The synthesis method of Methyl glycolate.
Oct 19,2023
Background
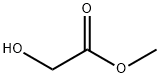
Methyl glycolate (MG), which contains both hydroxyl and ester groups, is recognized as a crucial fine chemical. Due to its resemblance in chemical properties to both alcohol and ester, MG can undergo a multitude of reactions such as carbonylation, hydrolysis, and oxidation. Not only does MG serve as a significant intermediate in the synthesis of biodegradable polymers, but it also acts as a pivotal platform molecule for the production of ethylene glycol and ethanol (EtOH)[1]. A highly promising method for producing glycolic acid with exceptional purity is catalytic hydrolysis of methyl glycolate.
Current situation
Currently, the production of MG relies exclusively on fossil resources and involves multi-step reactions and complicated separation processes. In addition, many traditional synthetic technologies, such as the carboxylation of formaldehyde and condensation of methyl formate with formaldehyde, have been applied for the synthesis of MG. However, disadvantages such as environmental pollution, harsh production process, and low stability of catalyst are obvious.
Cellulosic biomass
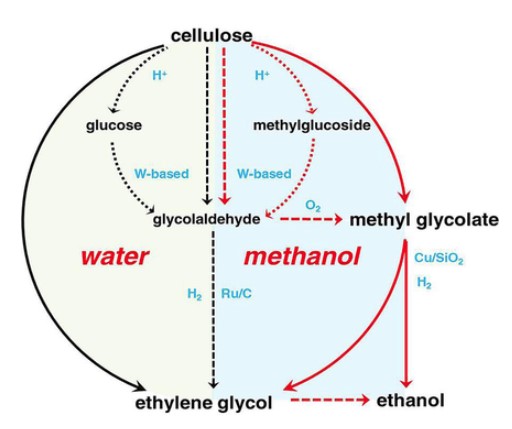
Xu et al. conceive that once MG can be produced from renewable lignocellulose, a new chemocatalytic, nonenzymatic route for the production of cellulosic EtOH will be available, that is highly desirable and even competitive to the current high-cost enzyme-catalyzed cellulosic EtOH. To mitigate the dependence on fossil resources, they reported a chemocatalytic conversion route from cellulosic biomass to methyl glycolate (MG), ethylene glycol (EG), and ethanol (EtOH). By using a tungsten‐based catalyst, cellulose is converted into MG with a yield as high as 57.7 C % in a one‐pot reaction in methanol at 240 °C and 1 MPa O2, and the obtained MG can be easily separated by distillation[2].
Ni3P catalyst
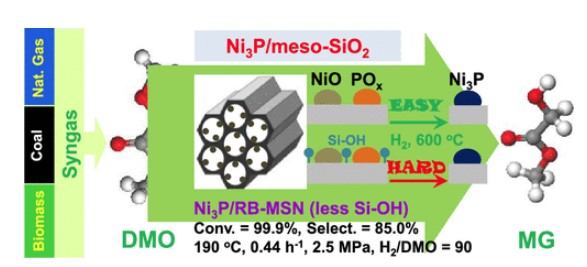
Zhao et al. prepared three meso-SiO2 (RB-MSN, HMS, and MCM-41) supported Ni3P catalysts via the impregnation method and subsequent H2-reduction treatment[3]. These catalysts could be used in gas-phase hydrogenation of dimethyl oxalate (DMO) to methyl glycolate (MG). The as-obtained catalysts show interesting meso-SiO2-type-dependent reactivity for this reaction, which is tightly linked with the support Si–OH amount rather than the support textural/structural properties and Ni3P grain size. The Ni3P/RB-MSN outperforms the two others, being capable of fully converting DMO with 85.0% MG selectivity and being stable for at least 500 h under mild conditions.
References
[1] Weng Y, et al. Conversion of glucose to methyl glycolate in subcritical methanol. Chemical Communications, 2023; 59: 4340-4343.
[2] Xu G, et al. Chemocatalytic Conversion of Cellulosic Biomass to Methyl Glycolate, Ethylene Glycol, and Ethanol. ChemSusChem, 2017; 10: 1390-1394.
[3] Zhao G, et al. High-Performance Ni3P/meso-SiO2 for Gas-Phase Hydrogenation of Dimethyl Oxalate to Methyl Glycolate. ACS Sustainable Chemistry & Engineering, 2021; 9: 16719–16729.
- Related articles
- Related Qustion
- Methyl glycolate: Overview, Conversion of Glucose to Methyl Glycolate and Safety Considerations Feb 23, 2024
Methyl glycolate, vital in industry and polymers, is synthesized from glucose through methanolysis. Safety precautions are crucial due to its combustible nature and irritant properties.
Dexamethasone is a synthetic glucocorticoids derived from cortisol. It has been widely used for the treatment of several diseases and in patients under chronic treatment.....
Oct 18,2023APIAntioxidant 565 is a versatile compound that exhibits strong antioxidant properties, with applications in various industries.....
Oct 19,2023APIMethyl glycolate
96-35-5You may like
Methyl glycolate manufacturers
- Methyl glycolate
-

- $0.00 / 1kg
- 2023-11-27
- CAS:96-35-5
- Min. Order: 1kg
- Purity: 0.99
- Supply Ability: 20 tons
- Methyl glycolate
-

- $30.00 / 1KG
- 2023-09-06
- CAS:96-35-5
- Min. Order: 50KG
- Purity: 99%
- Supply Ability: 500000kg
- Methyl glycolate
-

- $10.00 / 1KG
- 2023-04-11
- CAS:96-35-5
- Min. Order: 1KG
- Purity: 99.%
- Supply Ability: 10 ton