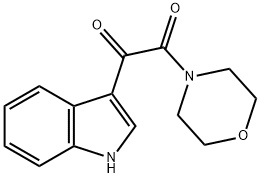
1-(1H-Indol-3-yl)-2-morpholin-4-yl-2-oxoethanone synthesis
- Product Name:1-(1H-Indol-3-yl)-2-morpholin-4-yl-2-oxoethanone
- CAS Number:5625-89-8
- Molecular formula:C14H14N2O3
- Molecular Weight:258.27

110-91-8
672 suppliers
$9.00/1g
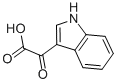
1477-49-2
124 suppliers
$10.00/100mg

5625-89-8
12 suppliers
$302.00/1g
Yield:5625-89-8 95%
Reaction Conditions:
Stage #1: 3-indolylglyoxylic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 0.0833333 h;Inert atmosphere;
Stage #2: morpholine in tetrahydrofuran;N,N-dimethyl-formamide;Inert atmosphere;
Steps:
4.1.1 General procedure for the amide coupling of 2-(Benzo[d]thiazol-2-ylthio)acetic acid with 1°/2° amines
General procedure: To the THF (5mL) and DMF (0.5mL) solution of 2-(Benzo[d]thiazol-2-ylthio)acetic acid (100 mg; mM) was added EDC.HCl (1.5molar equivalents) and HOBt (10mg). The reaction mixture was stirred for five minutes under nitrogen atmosphere at room temperature. Then 1.2 molar equivalents of amine (1°/2°) were added to the reaction mixture and the reaction mixture was continued to stir for further 12-16h. After the completion of the reaction monitored by TLC, the reaction mixture was quenched with excess of water (100mL) and extracted with ethylacetate (2×20mL). Combined organic layers were dried over anhydrous sodium sulfate and evaporated to obtain the target compound.
References:
Mir, Fauzia;Shafi, Syed;Zaman;Kalia, Nitin Pal;Rajput, Vikrant S.;Mulakayala, Chaitanya;Mulakayala, Naveen;Khan, Inshad A.;Alam [European Journal of Medicinal Chemistry,2014,vol. 76,p. 274 - 283]
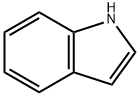
120-72-9
611 suppliers
$6.00/25g

5625-89-8
12 suppliers
$302.00/1g
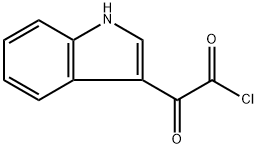
22980-09-2
72 suppliers
$39.00/1g

5625-89-8
12 suppliers
$302.00/1g

120-72-9
611 suppliers
$6.00/25g

79-37-8
460 suppliers
$17.67/10gm:

5625-89-8
12 suppliers
$302.00/1g