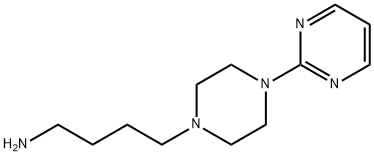
1-(2-Pyrimidinyl)-4-(4-aminobutyl)piperazine synthesis
- Product Name:1-(2-Pyrimidinyl)-4-(4-aminobutyl)piperazine
- CAS Number:33386-20-8
- Molecular formula:C12H21N5
- Molecular Weight:235.33
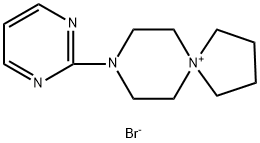
81461-73-6
33 suppliers
inquiry

33386-20-8
36 suppliers
$505.64/5MG
Yield: 99%
Reaction Conditions:
Stage #1:8-(2-Pyrimidinyl)-8-aza-5-azoniaspiro[4.5]decane Bromide with caesium carbonate in 5,5-dimethyl-1,3-cyclohexadiene at 130; for 1 h;
Stage #2: with iminodicarboxylic acid di-tert-butyl ester in 5,5-dimethyl-1,3-cyclohexadieneHeating;
Steps:
1 Example 1 - 4-(4-(pyrimidin-2-yl)piperazin- 1 -yl)butan- 1 -amine (5)
10.0 g of 8-(pyrimidin-2-yl)-5,8-diazaspiro[4,5]decan-5-ium bromide (11) (0.0334 moles), obtained according to US 4423049, is suspended in xylene (150 mL). 21.78 g of caesium carbonate (0.0668 moles) is then added. The resulting mixture is heated to 130°C and left under stirring for 60 minutes. 12.7 g of di-tert-butyl iminodicarboxylate (0.0584 moles) is then added and left under stirring until the reaction is complete. The mixture is cooled to about 80°C and filtered under vacuum, and the solid filtrate is washed with xylene (100 mL). 50 mL of 37% HC1 is added to the organic phase, and the resulting mixture is left under stirring for 10 min. The phases are then separated, and the organic phase is washed with a mixture of 50 mL of water and 5 mL of 37% HC1. 130 mL of dichloromethane is added to the aqueous acid phase and basified with 30% NaOH until pH = 13 is reached. The resulting mixture is left under stirring for 10 min., and the phases are separated. The aqueous phase is re-extracted with 200 mL of dichloromethane, and the combined organic phases are washed with 300 mL of water and 50 mL of brine, dried on sodium sulphate, filtered, and finally concentrated under vacuum to give 7.8 g of 4-(4-(pyrimidin-2-yl)piperazin-l-yl)butan-l-amine (5) (orange oil; yield 99%). (0046) 1H NMR (400 MHz, chloroform-d) d 8,17 (d, J = 4,7 Hz, 2H), 6,34 (t, J = 4,7 Hz, 1H), 3,76 - 3,63 (m, 4H), 2,60 (t, J = 6,9 Hz, 2H), 2,46 - 2,32 (m, 4H), 2,32 - 2,21 (m, 2H), 1,53 - 1,24 (m, 6H). (0047) 13C NMR (101 MHz, chloroform-d) d 161,55, 157,55, 109,65, 58,48, 53,02, 43,57, 42,01, 31,63, 24,18.
References:
PROCOS S.P.A.;COLOMBANO, Giampiero;BARBERO, Mauro;GIOVENZANA, Giovanni B;ROLETTO, Jacopo;PAISSONI, Paolo WO2020/148621, 2020, A1 Location in patent:Page/Page column 15-16

33386-14-0
0 suppliers
inquiry

33386-20-8
36 suppliers
$505.64/5MG
20980-22-7
349 suppliers
$5.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

33386-20-8
36 suppliers
$505.64/5MG
5394-18-3
279 suppliers
$5.00/1g

94021-22-4
117 suppliers
$15.00/5g

33386-20-8
36 suppliers
$505.64/5MG

20980-22-7
349 suppliers
$5.00/1g

5394-18-3
279 suppliers
$5.00/1g

33386-20-8
36 suppliers
$505.64/5MG