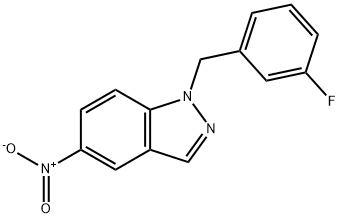
1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole synthesis
- Product Name:1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole
- CAS Number:529508-58-5
- Molecular formula:C14H10FN3O2
- Molecular Weight:271.25
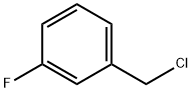
456-42-8
327 suppliers
$5.00/5g
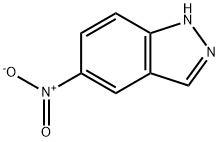
5401-94-5
364 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
55 suppliers
$108.00/1g
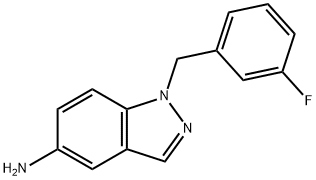
202197-31-7
23 suppliers
$463.00/1g
Yield:529508-58-5 33% ,202197-31-7 90%
Reaction Conditions:
with Ki;potassium carbonate in water;N,N-dimethyl-formamide;
Steps:
1.E E.
E. Preparation of 1-(3-Fluoro-benzyl)-1H-indazol-5-ylamine A mixture of 5-nitro-1H-indazole (8.15 gm, 50 mmole), m-fluoro-benzyl chloride (7.95 gm, 1.1 equiv), K2CO3 (7.59 gm, 1.1 equiv), and KI (8.47 gm, 1.02 equiv) in dry DMF (75 mL) was heated at 70° C. overnight. After cooling to RT, water (75 mL) was slowly added to give a precipitate that consisted of about a one to one mixture of isomers [HPLC Ret Time: 1.92 (1-substitued isomer vs. 2.03 (2-substituted isomer) YMC C18 S5 4.6*50 mm, 3 min gradient, 4 mL/min]. This was collected by filtration and washed with water. The solid was crystallized twice from acetone/water to afford the desired 1-(3-fluoro-benzyl)-5-nitro-1H-indazole (4.47 gm, 33%). A suspension of this material (3.00 gm, 11.1) and 10% Pd/C (3.00 gm) in EtOH (21 mL) was kept under an H2 atmosphere (balloon) for 24 hr. The catalyst was removed by filtration and the solvent was evaporated to leave the product as a solid (2.4 gm, 90%). 1H NMR (CDCl3): δ 3.61 (br s, 2H), 5.52 (s, 2H), 6.81-7.85 (m, 7H), 7.85 (s, 1H); MS: 242 (M+H)+; HPLC Ret Time: 1.03 min (YMC Xterra ODS S7, 3.0*50 mm column, 2 min gradient, 5 mL/min).
References:
US2003/186983,2003,A1
456-41-7
291 suppliers
$13.00/25g

5401-94-5
364 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
55 suppliers
$108.00/1g

456-41-7
291 suppliers
$13.00/25g

5401-94-5
364 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
55 suppliers
$108.00/1g
![2H-Indazole, 2-[(3-fluorophenyl)methyl]-5-nitro-](/CAS2/GIF/884874-01-5.gif)
884874-01-5
2 suppliers
inquiry

456-41-7
291 suppliers
$13.00/25g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
55 suppliers
$108.00/1g

456-41-7
291 suppliers
$13.00/25g

5401-94-5
364 suppliers
$14.00/5g
![1-[(3-Fluorophenyl)methyl]-5-nitro-1H-indazole](/CAS/GIF/529508-58-5.gif)
529508-58-5
55 suppliers
$108.00/1g
![1H-Benzimidazole, 2-[(3-fluorophenyl)methyl]-5-nitro-](/CAS/20180601/GIF/833474-48-9.gif)
833474-48-9
0 suppliers
inquiry