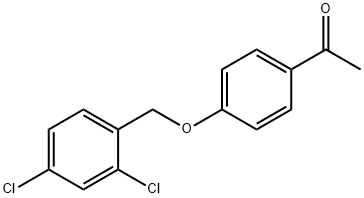
1-(4-[(2,4-DICHLOROBENZYL)OXY]PHENYL)-1-ETHANONE synthesis
- Product Name:1-(4-[(2,4-DICHLOROBENZYL)OXY]PHENYL)-1-ETHANONE
- CAS Number:61292-27-1
- Molecular formula:C15H12Cl2O2
- Molecular Weight:295.16
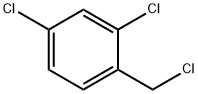
94-99-5
369 suppliers
$5.00/10g
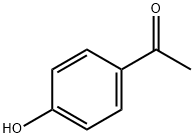
99-93-4
827 suppliers
$5.00/25g
![1-(4-[(2,4-DICHLOROBENZYL)OXY]PHENYL)-1-ETHANONE](/CAS/GIF/61292-27-1.gif)
61292-27-1
19 suppliers
$118.00/500mg
Yield: 82%
Reaction Conditions:
Stage #1:4-Hydroxyacetophenone with potassium tert-butylate in dimethyl sulfoxide at 5 - 10; for 0.666667 h;
Stage #2:2,4-Dichlorobenzyl chloride in dimethyl sulfoxide at 5 - 20;
Steps:
6.2. General procedure for the synthesis of compounds 2a-f and 14a-f
General procedure: To a stirred suspension of 4-hydroxy benzaldehyde/4-hydroxy acetophenone (1 mmol) in dimethyl sulfoxide (DMSO) (5 mL) at 5-10 °C was added potassium t-butoxide (1.2 mmol) was added in small portions in the period of 10 min. After stirring of 30 min, substituted benzyl/aryl halide (1 mmol) was added and then stirring was continued at room temperature for 4-5 h. The reaction mixture was quenched with ice cold water (5 mL) and extracted with ethyl acetate (3 × 5 mL), the combined organic layers were washed with water (3 × 5 mL) dried over Na2SO4 and concentrated under reduced pressure to obtain the crude product which was purified by column chromatography by using silica gel (60-120 mesh) and ethyl acetate: Hexane (4:94) as eluent to give the compounds (2a-f and 14a-f).
References:
Marrapu, Vijay K.;Chaturvedi, Vinita;Singh, Shubhra;Singh, Shyam;Sinha, Sudhir;Bhandari, Kalpana [European Journal of Medicinal Chemistry,2011,vol. 46,# 9,p. 4302 - 4310] Location in patent:experimental part