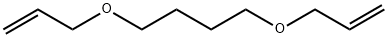
1,4-bis(allyloxy)butane synthesis
- Product Name:1,4-bis(allyloxy)butane
- CAS Number:1471-16-5
- Molecular formula:C10H18O2
- Molecular Weight:170.25
Yield:1471-16-5 68%
Reaction Conditions:
with sodium for 3 h;Reflux;
Steps:
2
General procedure: Sodium (4.8 g, 0.21 geatoms) was added to 20 ml of allylalcohol. While stirring, the desired dihalide compound (0.1 mol)was added over a 30 min period. The mixture was refluxed for 3 hand then taken up in ether; the ether layer was washed with waterand then the solvent was evaporated using a rotary evaporator. Theresidue was dried over anhydrous sodium sulfate, and finally theresidue was twice distilled under reduced pressure to obtain thedesired product.
References:
Baghersad, Mohammad Hadi;Habibi, Azizollah;Heydari, Akbar [Journal of Molecular Structure,2017,vol. 1130,p. 447 - 454]
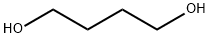
110-63-4
685 suppliers
$24.80/250g
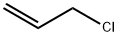
107-05-1
400 suppliers
$16.80/100ML
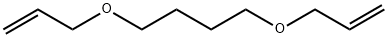
1471-16-5
4 suppliers
inquiry

