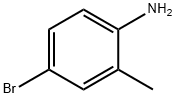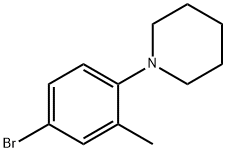
1-(4-Bromo-2-methylphenyl)piperidine synthesis
- Product Name:1-(4-Bromo-2-methylphenyl)piperidine
- CAS Number:1020180-19-1
- Molecular formula:C12H16BrN
- Molecular Weight:254.17
Yield:1020180-19-1 41.5%
Reaction Conditions:
with sodium carbonate in ethanol at 80; for 48 h;
Steps:
123.1
Following General Scheme 4 above, ethanol (5 ml_), 4-bromo-2-methanyl- analine(0.5g, 2.69mmol), 1 ,5-dibromopentane (0.68 g, 2.69 mmol) and Na2C03 (0.855 g, 8.06 mmol) were added sequentially to a 20-mL vial with a stirring bar. The vial was then sealed and heated to 80°C for 48hrs. After cooling down, the solid was filtered off and the filtrate was concentrated down in vacuo. The crude product was purified by column chromatography (silica gel, 0 to 30% ethyl acetate in hexanes) to give the title intermediate (310 mg, yield: 41.5%) LC- MS: m/z 254 (M+1 ).
References:
WO2012/37108,2012,A1 Location in patent:Page/Page column 187
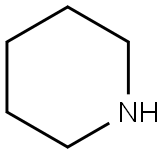
110-89-4
3 suppliers
$15.96/100ML
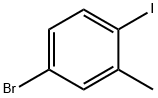
116632-39-4
202 suppliers
$8.00/5g

1020180-19-1
7 suppliers
inquiry