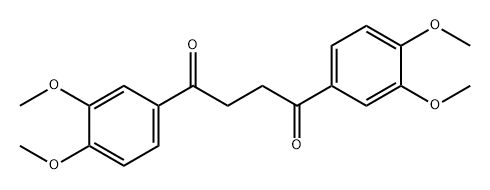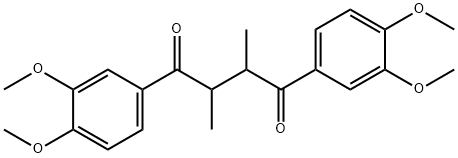
1,4-BUTANEDIONE synthesis
- Product Name:1,4-BUTANEDIONE
- CAS Number:4440-92-0
- Molecular formula:C22H26O6
- Molecular Weight:386.44
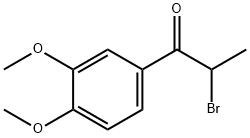
1835-05-8
34 suppliers
$470.39/250mg
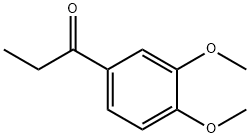
1835-04-7
66 suppliers
$21.00/100mg

4440-92-0
8 suppliers
inquiry
Yield:4440-92-0 95%
Reaction Conditions:
Stage #1: ethyl 3,4-dimethoxyphenyl ketonewith potassium tert-butylate in tetrahydrofuran at 0 - 7; for 0.25 h;Inert atmosphere;
Stage #2: in tetrahydrofuran;N,N-dimethyl-formamide at 18; for 0.283333 h;
Stage #3: 2-bromo-1-(3,4-dimethoxyphenyl)propan-1-one in tetrahydrofuran;N,N-dimethyl-formamide at -70 - -55; for 0.666667 h;
Steps:
4.1.3 1,4-Bis(3,4-dimethoxyphenyl)-2,3-dimethylbutane-1,4-dione (3)
As outlined in Fig. 3, to a dry 5 L, 3-necked round bottom flask (equipped with an overhead magnetic stir drive, addition funnel, thermocouple and N2 inlet and outlet) was added solid 97% t-BuOK (103 g, 898.5 mmol, corrected for purity), followed by THF (615 mL). The solution was cooled to 0-1 °C with an ice-water bath. A solution of propiophenone 7 (170 g, 876.3 mmol) in THF (340 mL) was added in portions over 15 min while keeping the internal temperature <7°C giving a white/yellow-white slurry. After 15 min the mixture was warmed to 18 °C and DMF (850 mL) was added via the addition funnel over 2 min giving a clear yellow/orange solution. After 15 min, the reaction mixture was cooled to -70 °C using a dry ice/acetone bath. With vigorous stirring, a solution of α-bromoketone 8 (240 g, 876.3 mmol) in 2:1 THF-DMF (510 mL) was added in portions over 25 min while maintaining an internal temperature between -60 °C and -55 °C. After an additional 15 min at -60 °C, the reaction was complete as determined by LC/MS analysis. The reaction was quenched at -60 °C with water (900 mL) containing 70 mL 1 M HCl and the reaction was warmed to 18-20 °C over 1 h. The bulk of THF was removed in vacuo (1415 mL solvent removed) and the resulting mixture was extracted with CH2Cl2 (1.5 L). The organic layer was separated and the aqueous layer (pH 2-3) was back extracted twice with CH2Cl2 (2 × 400 mL). The combined CH2Cl2 was washed with water (425 mL). The bulk of the CH2Cl2 (1550 mL) was removed in vacuo to give crude product 3, which was further purified from methanol to give the expected product 321.7 g (95% yield, purity>98% as determined by HPLC). 1H NMR (CDCl3, 400 MHz) δ: 1.31 (d, J = 7.1 Hz, 2CH3, 6H), 3.55 (m, 2H), 3.85 (s, 4OCH3, 12H), 6.98 (d, 2H, J = 6.9 Hz), 7.24-719 (m, 4H). 13C NMR (CDCl3, 100 MHz) δ: 11.82, 42.11, 58.87, 110.14, 110.23, 131.33, 149.44, 150.11, 198.22. LC/MS (m/z = 386.4).
References:
Li, Xu;Jiang, Jian-Hong;Chen, Qingqi;Xiao, Sheng-Xiong;Li, Chuan-Hua;Gu, Hui-Wen;Zhang, Hui;Hu, Ji-Lin;Yao, Fei-Hong;Li, Qiang-Guo [European Journal of Medicinal Chemistry,2013,vol. 62,p. 605 - 613]

1835-04-7
66 suppliers
$21.00/100mg

4440-92-0
8 suppliers
inquiry
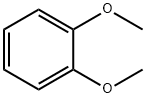
91-16-7
537 suppliers
$13.00/25g

4440-92-0
8 suppliers
inquiry
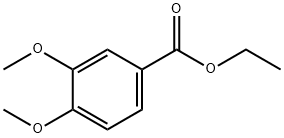
3943-77-9
55 suppliers
$35.00/5g

4440-92-0
8 suppliers
inquiry