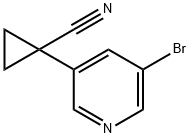
1-(5-broMopyridin-3-yl)cyclopropanecarbonitrile synthesis
- Product Name:1-(5-broMopyridin-3-yl)cyclopropanecarbonitrile
- CAS Number:1272357-22-8
- Molecular formula:C9H7BrN2
- Molecular Weight:223.07
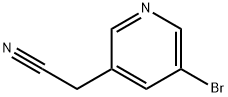
39891-08-2
66 suppliers
$65.00/100mg

107-04-0
268 suppliers
$9.00/1g

1272357-22-8
16 suppliers
inquiry
Yield:1272357-22-8 57%
Reaction Conditions:
with benzyl-triethyl-ammonium chloride;sodium hydroxide in lithium hydroxide monohydrate at 60; for 16 h;
Steps:
1.1.18
To a stirred suspension of 2-(5-bromopyridin-3-yl)acetonitrile (1.0 g, 5.08 mmol) in 50% aq. NaOH (20 mL) was added l-bromo-2 -chloroethane (0.34 mL, 5.33 mmol) followed by N-benzyl-N,N- diethylethanaminium chloride (23.12 mg, 0.1 mmol). The resulting mixture was stirred at 60 °C for 16 h. After this time the mixture was diluted with water (200 mL) and extracted with DCM (2 x 200 mL). The combined organic extracts were dried (MgSOft. filtered and concentrated at reduced pressure. The residue was purified by Biotage Isolera column chromatography (50 g KP-Sil column, eluting with a gradient of eluents: 0-40% EtOAc in heptane) to give the title compound (647 mg, 57% yield) as an off-white powder. LCMS (Method D) rt = 0.98 min, MS (ESIPos) m/z=222.8 & 224.75 [M+H]+
References:
WO2022/128850,2022,A1 Location in patent:Paragraph 0223; 0226; 0246
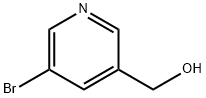
37669-64-0
215 suppliers
$12.00/1g

1272357-22-8
16 suppliers
inquiry
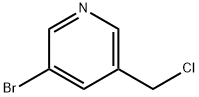
120277-69-2
63 suppliers
$48.00/100mg

1272357-22-8
16 suppliers
inquiry