
1,9-DIAMINONONANE synthesis
- Product Name:1,9-DIAMINONONANE
- CAS Number:646-24-2
- Molecular formula:C9H22N2
- Molecular Weight:158.28

1675-69-0
45 suppliers
$157.25/25 mL

646-24-2
102 suppliers
$35.00/1g
Yield:646-24-2 88%
Reaction Conditions:
with ammonia;hydrogen;sodium hydroxide;Raney nickel in water at 130; under 22502.3 Torr; for 6 h;Product distribution / selectivity;Autoclave;
Steps:
14
300 g (2 mol) of azelonitrile obtained in example 13 are charged, with 9 g of Raney nickel, to a clean and dry 500 cm3 autoclave. This autoclave is closed and the gas phase is purged with nitrogen. 17 g of ammonia (1 mol, i.e. 0.25 mol of NH3/mole of CN functional group) and 0.6 g of 50% by weight sodium hydroxide in water are subsequently introduced at ambient temperature. The reaction medium is placed under stirring and then the hydrogen is introduced so that the total pressure is 30 bar at 130° C.After reacting for 6 hours, cooling is carried out and the catalyst is filtered off at a temperature of 60° C. The crude diamine is distilled conventionally under reduced pressure. The 1,9-diaminononane is obtained with a purity of 99.2% and a yield of 88%. The diamine does not comprise impurity such as ethyl-1,9-diamino-nonane.
References:
Ceca S.A. US2011/190541, 2011, A1 Location in patent:Page/Page column 5
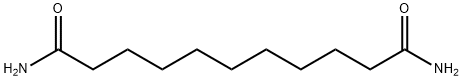
73154-82-2
0 suppliers
inquiry

646-24-2
102 suppliers
$35.00/1g
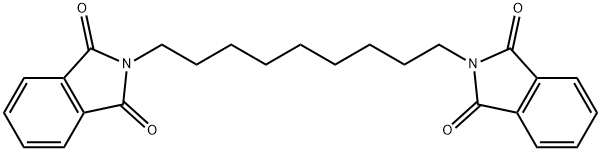
37830-04-9
0 suppliers
inquiry

646-24-2
102 suppliers
$35.00/1g
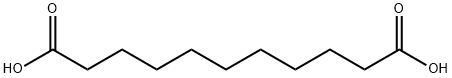
1852-04-6
275 suppliers
$5.00/10g

646-24-2
102 suppliers
$35.00/1g
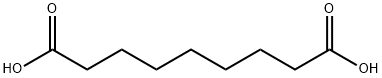
123-99-9
694 suppliers
$14.00/5g

646-24-2
102 suppliers
$35.00/1g