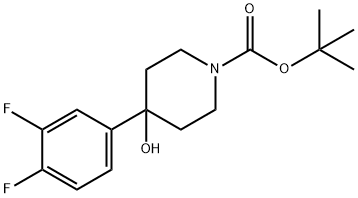
1-BOC-4-(3,4-DIFLUOROPHENYL)-4-HYDROXYPIPERIDINE synthesis
- Product Name:1-BOC-4-(3,4-DIFLUOROPHENYL)-4-HYDROXYPIPERIDINE
- CAS Number:871112-36-6
- Molecular formula:C16H21F2NO3
- Molecular Weight:313.34
Yield:871112-36-6 54%
Reaction Conditions:
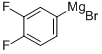
Stage #1: N-tert-butyloxycarbonylpiperidin-4-one;3,4-difluorophenylmagnesium bromide in tetrahydrofuran at -78 - 20; for 18 h;Inert atmosphere;
Stage #2: with ammonium chloride in tetrahydrofuran at -78;
Steps:
2.2
Scheme 2: Preparation of (S)-l-(4-(3,4-difluorophenyl)-4-hydroxypiperidin-l- yl)-2-(4-Isobutyl phenyl)propan-l-one (7a) Preparation of tert-butyl 4-(3,4-difluorophenyl)-4-hydroxypiperidine-l- carboxylate (110). To a stirred solution of tert-butyl 4-oxopiperidine-l-carboxylate (108, 250 mg, 1.255 mmol) in anhydrous THF (5 mL), was added (3,4- difluorophenyl)magnesium bromide (0.5 M in THF) (109, 3.8 mL, 1.882 mmol) dropwised under nitrogen at -78 °C. The reaction mixture was then stirred at rt under nitrogen for 18 h. It was then stopped, quenched with sat. ammonium chloride solution at -78 °C. The reaction was extracted with EtOAc (3 x 50 mL) and the EtOAc extracts were washed with brine (50 mL), dried over sodium sulphate, filtered, and concentrated under vacuum. Purification of the residue by flash column chromatography (silica gel, 1 :5 EtOAc/Hexanes) provided 110 (212 mg, 54%): 1H NMR (300 MHz, CDC13) δ 7.34-7.29 (m, 1H), 7.19-7.10 (m, 2H), 4.04 (br s, 1H), 3.20 (t, J= 9.0 Hz, 2H), 1.93 (t, J= 8.4 Hz, 2H), 1.71-1.65 (m, 3H), 1.58 (s, 1H), 1.48 (s, 9H)
References:
WO2011/103126,2011,A1 Location in patent:Page/Page column 38-39