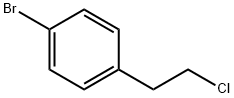
1-BROMO-4-(2-CHLOROETHYL)BENZENE synthesis
- Product Name:1-BROMO-4-(2-CHLOROETHYL)BENZENE
- CAS Number:23386-17-6
- Molecular formula:C8H8BrCl
- Molecular Weight:219.51
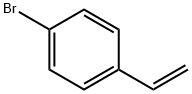
2039-82-9
266 suppliers
$10.00/1g

23386-17-6
5 suppliers
inquiry
Yield:23386-17-6 34%
Reaction Conditions:
with 4-Methoxybenzenethiol;10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate;2,6-lutidine hydrochloride in chloroform;2,2,2-trifluoroethanol; for 48 h;Irradiation;Inert atmosphere;
Steps:
Methods
General procedure: The general procedure for the anti-Markovnikov hydrochlorination of styrenesusing 2,6-lutidine hydrochloride (Method B) was as follows. In a typical experiment,a flame-dried 2 dram vial was equipped with a magnetic stir bar and 9-mesityl-10-methylacridinium tetrafluoroborate (1a, 30.8 mg, 0.0771 mmol, 5.0 mol%). The vialwas placed under an inert atmosphere and 2,6-lutidine hydrochloride (443 mg, 3.08mmol, 2.0 equiv.) was added. The solvent CHCl3/TFE (2.5:1 (vol:vol), 2.8 ml) wasadded to a concentration of ~0.55 M. Liquid substrate (β-methylstyrene 3a, 200 μl,1.54 mmol, 1 equiv.) was added via microsyringe after the solvent. The hydrogenatom donor, 4-methoxythiophenol (2b, 37.9 μl, 0.308 mmol, 20 mol%), was addedvia microsyringe. The vial was sealed with a Teflon-coated septum cap, and thereaction mixture was irradiated (450 nm) for the time indicated. Upon completion,saturated aqueous sodium bicarbonate was added carefully and the two phases wereallowed to separate. The organic phase was collected, and the aqueous phase wasextracted with two portions of dichloromethane. The combined organic portionswere passed through a short plug of SiO2. The solvent was removed under reducedpressure. NMR yields were taken by integration against a known quantity ofhexamethyldisiloxane (HMDS) in CDCl3. The final products were isolated by silicagel chromatography using the conditions listed.
References:
Wilger, Dale J.;Grandjean, Jean-Marc M.;Lammert, Taylor R.;Nicewicz, David A. [Nature Chemistry,2014,vol. 6,# 8,p. 720 - 726]
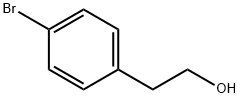
4654-39-1
272 suppliers
$5.00/1g

23386-17-6
5 suppliers
inquiry
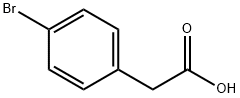
1878-68-8
472 suppliers
$6.00/10g

23386-17-6
5 suppliers
inquiry

4654-39-1
272 suppliers
$5.00/1g
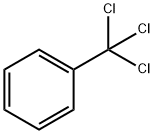
98-07-7
321 suppliers
$13.00/25g
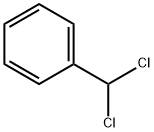
98-87-3
4 suppliers
$30.00/25g

23386-17-6
5 suppliers
inquiry
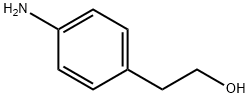
104-10-9
335 suppliers
$10.00/1g

23386-17-6
5 suppliers
inquiry