
1-BROMO-7-METHYLOCTANE synthesis
- Product Name:1-BROMO-7-METHYLOCTANE
- CAS Number:54088-99-2
- Molecular formula:C9H19Br
- Molecular Weight:207.15
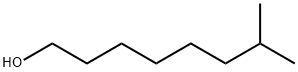
2430-22-0
44 suppliers
$180.00/250mg

54088-99-2
16 suppliers
inquiry
Yield:54088-99-2 74%
Reaction Conditions:
with N-Bromosuccinimide;triphenylphosphine in DMF (N,N-dimethyl-formamide) at 20; for 0.5 h;
Steps:
14.14b 14b. 1-Bromo-7-methyl-octane
To a solution of 7-methyl-octan-1-ol (1.20 g, 8.33 mmol) in DMF (20 ML) was added triphenylphosphine (2.43 g, 9.26 mmol).The solution was cooled to 0° C. and NBS (1.60 g, 8.99 mmol) was added in portions.After stirring for 30 min at room temperature, the reaction was quenched with methanol (1 ML).The solution was diluted with ether (100 ML), washed with water, saturated aqueous NaHCO3 and brine successively.The organic layer was dried and concentrated.The residue was purified by flash chromatography on silica gel, eluding with hexane to afford 1-bromo-7-methyl-octane (1.28 g, 74%) as a colorless oil: 1H NMR (300 MHz, CDCl3) δ 3.41 (2H, t, J=6.6 Hz), 1.85 (2H, m), 1.52 (1H, m), 1.42 (2H, m), 1.28 (4H, m), 1.17 (2H, m), 0.87 (3H, s), 0.85 (3H, s); 13C NMR (75 MHz, CDCl3) δ 39.16, 34.34, 33.14, 29.34, 28.52, 28.24, 27.51, 22.94.
References:
US2003/225142,2003,A1 Location in patent:Page 12

463964-83-2
0 suppliers
inquiry

54088-99-2
16 suppliers
inquiry

629-30-1
247 suppliers
$8.00/1g

54088-99-2
16 suppliers
inquiry

10160-24-4
185 suppliers
$19.00/1g

54088-99-2
16 suppliers
inquiry
30515-28-7
164 suppliers
$7.00/250mg

54088-99-2
16 suppliers
inquiry