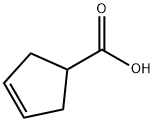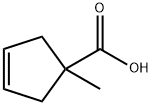
1-methylcyclopent-3-ene-1-carboxylic acid synthesis
- Product Name:1-methylcyclopent-3-ene-1-carboxylic acid
- CAS Number:124346-92-5
- Molecular formula:C7H10O2
- Molecular Weight:126.15
Yield:124346-92-5 100%
Reaction Conditions:
Stage #1: 3-cyclopentene-1-carboxylic acidwith lithium diisopropyl amide in tetrahydrofuran;n-heptane;ethylbenzene at -30 - 25; for 15 h;
Stage #2: methyl iodide in tetrahydrofuran;n-heptane;ethylbenzene at -30 - 25; for 2 h;
Steps:
40.40B Step 40B.
1-Methylcyclopent-3-ene-1-carboxylic Acid
To a solution of LDA (2M in THF/heptane/ethyl benzene, 446 mL) in THF (800 mL) was added dropwise a solution of cyclopent-3-ene-1-carboxylic acid (40 g, 357 mmol) in THF (200 mL) maintaining a temperature of -30° C.
The mixture was warmed to 25° C. and stirred for 15 h.
The mixture was then cooled to -30° C. and iodomethane (50.6 g, 22.2 mL, 357 mmol) was added.
After 2 h at 25° C., the mixture was quenched with 3M HCl and extracted with EtOAc (3*1 L).
The combined organic extracts were dried (Na2SO4) and filtered.
The filtrate was concentrated under reduced pressure and the residue was purified by silica gel column chromatography (EtOAc) to afford 45 g (100%) of the title compound as an oil. 1H NMR (400 MHz, CDCl3) δ 5.64 (s, 2H), 2.98 (d, J=14.7 Hz, 2H), 2.28 (d, J=14.7 Hz, 2H), 1.36 (s, 3H).
References:
US2020/95239,2020,A1 Location in patent:Paragraph 0664; 0666
84646-68-4
52 suppliers
$45.00/50mg

124346-92-5
5 suppliers
inquiry
58101-60-3
152 suppliers
$6.00/1g

124346-92-5
5 suppliers
inquiry