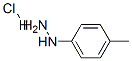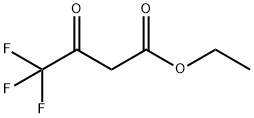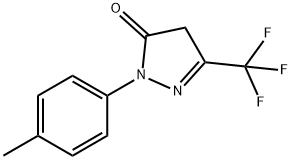
1-p-tolyl-3-(trifluoromethyl)-1H-pyrazol-5(4H)-one synthesis
- Product Name:1-p-tolyl-3-(trifluoromethyl)-1H-pyrazol-5(4H)-one
- CAS Number:63695-47-6
- Molecular formula:C11H9F3N2O
- Molecular Weight:242.2
Yield:-
Reaction Conditions:
with sodium acetate in acetic acid;Reflux;Inert atmosphere;
Steps:
General procedure for the synthesis of the target compounds 10a-g and 11a-g
General procedure: Acetylacetic ether or trifluoroacetyl ethyl acetate (25 mmol) were added to a mixture solution of 5a-g (25 mmol) and sodium acetate (26 mmol) in acetic acid (20 mL). The reaction mixture was stirred at reflux temperature for 5-10 h. After cooling, the mixture was added to a saturated solution of NaHCO3 until its pH value is adjusted to 7 and extracted with ethyl acetate for three times. The combined organic layer was then dried, filtered, concentrated and the residue was purified by column chromatography (PE/EtOAc = 6:1, v/v) to give light yellow solid 6a-g and 7a-g in 50-70% yield.
References:
Sheng, Xiao;Hua, Kai;Yang, Chunyu;Wang, Xiaoli;Ji, Hui;Xu, Jinyi;Huang, Zhangjian;Zhang, Yihua [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 17,art. no. 22880,p. 3535 - 3540]