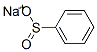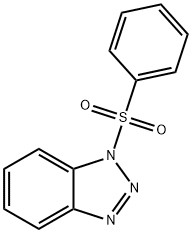
1-(PHENYLSULFONYL)-1H-BENZOTRIAZOLE synthesis
- Product Name:1-(PHENYLSULFONYL)-1H-BENZOTRIAZOLE
- CAS Number:4106-18-7
- Molecular formula:C12H9N3O2S
- Molecular Weight:259.28
Yield:4106-18-7 97%
Reaction Conditions:
with iodine in water;ethyl acetate at 20; for 3 h;Mechanism;Reagent/catalyst;Solvent;Time;
Steps:
A typical procedure for the catalytic N-sulfonylation of benzotriazoles using I2 as catalyst includes: in EtOAc-H2O (10:1) mixture solvent (2 mL), benzotriazole 1a (0.3 mmol), sodium benzenesulfinate 2a (0.9 mmol), I2 (0.06 mmol) were added successively.
The suspension mixture was vigorously stirred at room temperature for 3 h.
Upon completion, the reaction was quenched by addition of sat. aq. Na2S2O3 (2 mL), basified with sat. aq. Na2CO3 (8 mL) and H2O (5 mL).
The mixture was extracted with CH2Cl2 (3* 5 mL) and the combined organic phase was dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure.
The residue was then purified by TLC technique (petroleum ether/ethyl acetate = 3:1, v/v) to furnish 1-phenylsulfonylbenzotriazole 3a
[28]
in 97% yield.
Compound 3a: Pale yellow solid; mp 122-123 °C; 1H NMR (500 MHz, CDCl3): δ 8.15-8.11 (m, 3H), 8.09 (d, 1H, J = 8.4 Hz), 7.70-7.64 (m, 2H), 7.57-7.53 (m, 2H), 7.52-7.47 (m, 1H); 13C NMR (125 MHz, CDCl3): δ 145.4, 137.0, 135.2, 131.6, 130.3, 129.6, 127.8, 125.9, 120.5, 111.9; IR (film, cm-1): ν 3094, 3069, 1586, 1480, 1386, 1194, 955, 726, 589; MS (EI, m/z, %): 260 (M + 1, 100).
References:
Wu, Si-Xue;Zhang, Yi-Kun;Shi, Hong-Wei;Yan, Jie [Chinese Chemical Letters,2016,vol. 27,# 9,p. 1519 - 1522]
95-14-7
837 suppliers
$6.00/100g
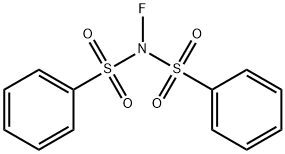
133745-75-2
392 suppliers
$5.00/1g

4106-18-7
11 suppliers
$264.00/1g
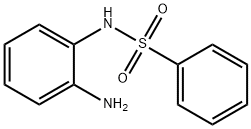
43200-31-3
7 suppliers
inquiry

4106-18-7
11 suppliers
$264.00/1g
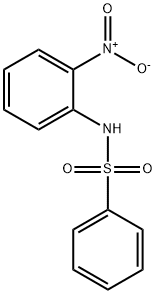
6933-51-3
9 suppliers
inquiry

4106-18-7
11 suppliers
$264.00/1g