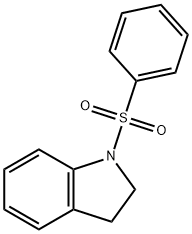
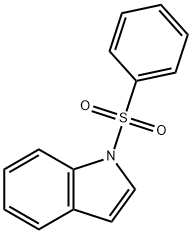
1-(phenylsulfonyl)indoline synthesis
- Product Name:1-(phenylsulfonyl)indoline
- CAS Number:81114-41-2
- Molecular formula:C14H13NO2S
- Molecular Weight:259.32
Yield:81114-41-2 97%
Reaction Conditions:
with anhydrous potassium trimethylsilanolate in water monomer at 20; for 0.5 h;Inert atmosphere;Concentration;
Steps:
14.4.2 14.4 Preparation of 1 -(phenylsulfonyl)indoline
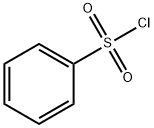
General procedure: Under an argon atmosphere, a base (2.97 mmol) and the aqueous oligosaccharide solution (2 wt % HPMC, in degassed Millipore water) were weighed into a 5 mL microwave vial containing a magnetic stir bar and a Teflon-lined septum. An amine (0.99 mmol) and subsequently a sulfonyl chloride (1.98 mmol) were added to the vigorously stirred reaction mixture at room temperature. Stirring was continued for the indicated time at the indicated temperature. The reaction mixture was diluted with ethyl acetate, the solids were filtered and the aqueous phase was extracted 3x with ethyl acetate. The combined organic extracts were dried with magnesium sulfate, filtered and concentrated in vacuo. The crude product was purified by flash chromatography on silica gel.; Following the general procedure using potassium trimethylsilanolate (380 mg, 2.97 mmol), indoline (119 mg, 0.99 mmol), benzenesulfonyl chloride (220 mg, 1.20 mmol) and 3 ml of aqueous oligosaccharide solution (2 wt % HPMC, 4-6 cps, in degassed Millipore water), the reaction was allowed to stir for 30 min room temperature. LC-MS and TLC indicated that the reaction was already completed after 5 min. After column chromatography (40-100 % n-heptane - dichloromethane), the product was obtained as a white crystalline material (262 mg, 97 %, 96% purity). ESI-MS: m/z (%): 260.20 (100, [M+H]+).1H NMR (600MHz, CDC13): δ [ppm]: 7.80 (m, 2H), 7.65 (m, 1H), 7.55 (m, 1H), 7.45 (m, 2H), 7.20 (m, 1H), 7.10 (m, 1H), 7.00 (m, 1H), 3.95 (m, 2H), 2.90 (m, 2H).
References:
WO2017/129796,2017,A1 Location in patent:Page/Page column 247; 248
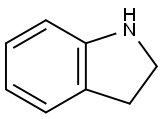
496-15-1
425 suppliers
$8.00/10g

81114-41-2
12 suppliers
inquiry

77198-99-3
10 suppliers
inquiry

81114-41-2
12 suppliers
inquiry
40899-71-6
147 suppliers
$5.00/1g

81114-41-2
12 suppliers
inquiry