
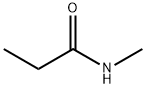
1-Propanamine, N-methyl-, hydrochloride synthesis
- Product Name:1-Propanamine, N-methyl-, hydrochloride
- CAS Number:65845-60-5
- Molecular formula:C4H11N.ClH
- Molecular Weight:109.5978
1187-58-2
123 suppliers
$66.00/5mL

65845-60-5
7 suppliers
inquiry
Yield:65845-60-5 89%
Reaction Conditions:
Stage #1: N-methylpropanamidewith bis(cyclopentadienyl)dihydrozirconium;4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane at 20; under 760.051 Torr; for 12 h;Inert atmosphere;
Stage #2: with hydrogenchloride in diethyl ether;Inert atmosphere;
Steps:
53
The bis(cyclopentadiene) zirconium dihydride (denoted as Cp2ZrH2, 0.01mmol, 2.23mg), N-methyl propionamide (denoted as 3j, 0.2mmol, 17.4mg) and pinacol borane (denoted as HBpin , 0.6mmol, 87μL), stirred for 12h at room temperature under nitrogen (1atm) atmosphere, treated with hydrogen chloride in ether solution to obtain the hydrochloride compound of formula 4j (brown solid,N-methylprop-1-amine hydrochloride). The isolated yield was 89%.
References:
CN112299938,2021,A Location in patent:Paragraph 0170-0172