N-[[[1-Methyl-1-(hydroxymethyl)ethyl]amino]thiocarbonyl]benzamide synthesis
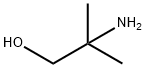
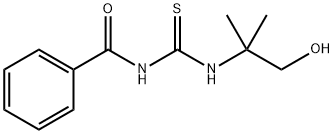
- Product Name:N-[[[1-Methyl-1-(hydroxymethyl)ethyl]amino]thiocarbonyl]benzamide
- CAS Number:1007232-81-6
- Molecular formula:C12H16N2O2S
- Molecular Weight:252.33
Yield:1007232-81-6 68%
Reaction Conditions:
in tert-butyl methyl ether at 18 - 25; for 1 h;Solvent;
Steps:
1-4 Example 3
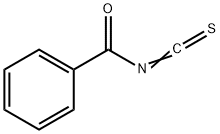
a method for synthesizing 1-benzoyl-3-(2-hydroxy-1,1-dimethylethyl)thiourea, equipped with mechanical stirring, a thermowell, 90 g of methyl tert-butyl ether was added to a 500 ml four-port reactor of a reflux condenser. 2-Amino-2-methylpropanol 28.2 g (0.3 mol). Maintain a temperature of about 19 °C with a water bath. Dissolve 19.5 g (0.1 mol) of benzoyl isothiocyanate50 g of methyl tert-butyl ether was added dropwise to the reaction system. The dropping process controls the temperature at 18-20 °C. After the completion of the dropwise addition, the mixture was stirred at room temperature at 25 °C for 1 h. The crude product was obtained by rotary distillation of methyl tert-butyl ether, the purity was 85%, and the crude yield was 96%. the crude product was added to 300ml water, stirred for 0.5h filter 60 °C and dried to give the product, and finally with 40ml of ethyl acetate and petroleum ether 40ml crude product was dried to obtain a purity greater than 99% of eligible product, yield 68 %.
References:
CN101525310,2019,B Location in patent:Paragraph 0012-0025