1-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-4-methylcyclohexane-1-carboxylic acid synthesis
- Product Name:1-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-4-methylcyclohexane-1-carboxylic acid
- CAS Number:1013096-03-1
- Molecular formula:C23H27NO4S2
- Molecular Weight:445.59
![3-Oxazolidinecarboxylic acid, 4-[[(1,1-dimethylethyl)dithio]methyl]-5-oxo-, 9H-fluoren-9-ylmethyl ester, (4R)-](/CAS/20210305/GIF/1013096-02-0.gif)
1013096-02-0
0 suppliers
inquiry
![1-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-4-methylcyclohexane-1-carboxylic acid](/CAS/20200119/GIF/1013096-03-1.gif)
1013096-03-1
6 suppliers
inquiry
Yield:1013096-03-1 72%
Reaction Conditions:
with chlorotriisopropylsilane;trifluoroacetic acid in chloroform at 20; for 16 h;
Steps:
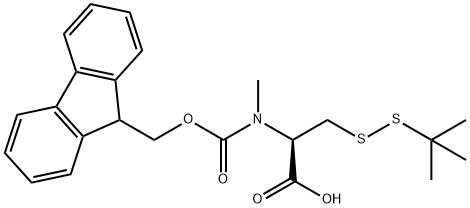
Compound 2.28 (i?)-2-((((9H-fluoren-9-yl)metlioxy)carbonyl)(n etliyl)an ino)-3- (tertbutyldisulfanyl) propanoic acid:
To a room temperature stirring solution of 256 mg (0.57 mmol) 2.27 in 3 mL chloroform under natural atmosphere was added 1.18 mL (5.7 mmol) triisopropyl silane followed by the rapid addition of 1.4 mL (2.5 mL/mmol) trifluoroacetic acid. The reaction was stirred at room temperature for 16 h and then concentrated. The oil was dissolved in 20 mL DCM and concentrated. This was repeated three consecutive times. After impregnation onto a gravity silica gel column, 2.28 was purified via isocratic column chromatography using ethyl acetate (40%) and hexanes (60%) to provide 320 mg (72%) 2.28 as a white solid in a 1.5: 1.0 conformer ratio as reported previously.99 1H NMR (300 MHz, CDC13) - 7.78 (d, J= 7.2 Hz, 2H), 7.60 (dd, J= 17.1, 7.7 Hz, 2H), 7.46 27.38 (m, 1H), 7.33 (td, J= 7.3, 4.9 Hz, 2H), 5.49 - 5.18 (m, 1H), 4.76 - 4.36 (m, 1H), 4.28 (m 1H), 3.85 - 3.70 (m, 0.5H), 3.58 (m, 0.5H), 3.40 - 3.10 (m, 0.5H), 3.03 (s, 3H), 1.27 (s, 9H). ESI-LRMS m/z = 468 [M+Na]+.
References:
WO2013/16531,2013,A2 Location in patent:Page/Page column 67
73724-43-3
129 suppliers
$28.00/1G
![1-({[(9H-fluoren-9-yl)methoxy]carbonyl}amino)-4-methylcyclohexane-1-carboxylic acid](/CAS/20200119/GIF/1013096-03-1.gif)
1013096-03-1
6 suppliers
inquiry