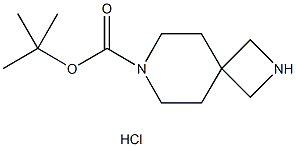
tert-Butyl2,7-diazaspiro[3.5]nonane-7-carboxylatehydrochloride synthesis
- Product Name:tert-Butyl2,7-diazaspiro[3.5]nonane-7-carboxylatehydrochloride
- CAS Number:1023301-84-9
- Molecular formula:C12H22ClN2O2-
- Molecular Weight:261.7683
929302-00-1
11 suppliers
inquiry
![tert-Butyl2,7-diazaspiro[3.5]nonane-7-carboxylatehydrochloride](/CAS2/GIF/1023301-84-9.gif)
1023301-84-9
119 suppliers
$14.00/100mg
Yield:-
Reaction Conditions:
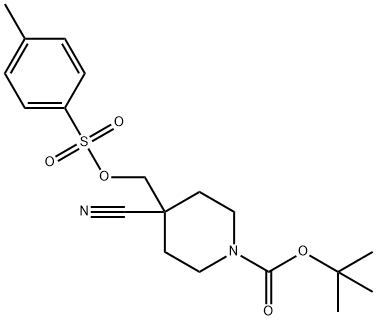
Stage #1: tert-butyl 4-cyano-4-({[(4-methylphenyl)sulfonyl]oxy}methyl)piperidine-1-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Stage #2: with sodium hydroxide in tetrahydrofuran;water; for 0.5 h;
Stage #3: with hydrogenchloride in methanol;diethyl ether;
Steps:
Preparation of compound 4THF (40 L) was added to LiAlH4 (810 mg, 2.13 mol) at 0 0C under N2. Then a solution of compound 3 (5 g, 1.37 mol) in 30 L of THF was added dropwise at rt over 4 h. After the addition, the mixture was stirred overnight. To the mixture was added dropwise 1.6 mL of 10% aq. NaOH and 0.8 mL of H2O and stirred for 0.5 h and filtered. The cake was washed with DCM. The filtrate was concentrated, the residue was dissolved in DCM, dried over Na2SO4 and concentrated. 2 mL of MeOH was added, followed by 5 L of ether. The mixture was adjusted to pH 6 with HCl/MeOH. The precipitate was filtered and washed with ether to give 1.70 g of compound 4. 1H NMR (CDCl3, HCl salt) δ: 9.71 (br, 2 H), 3.83 (t, 4 H), 3.34- 3.31 (m, 4 H), 1.86 - 1.83 (m, 4 H)3 1.43 (9 H).
References:
WO2007/30061,2007,A1 Location in patent:Page/Page column 92; 93