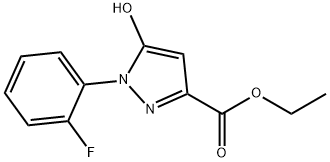
1-(2-fluoro-phenyl)-5-hydroxy-1H-pyrazole-3-carboxylic acid ethyl ester synthesis
- Product Name:1-(2-fluoro-phenyl)-5-hydroxy-1H-pyrazole-3-carboxylic acid ethyl ester
- CAS Number:1034303-23-5
- Molecular formula:C12H11FN2O3
- Molecular Weight:250.23
Yield:1034303-23-5 84%
Reaction Conditions:
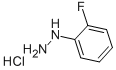
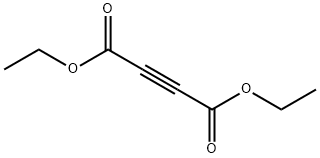
Stage #1: (2-fluorophenyl)hydrazine hydrochloride;acetylenedicarboxylic acid diethyl esterwith potassium carbonate in ethanol at 20; for 4 h;Heating / reflux;
Stage #2: with hydrogenchloride in ethanol;water; for 1 h;
Steps:
192 Ethyl 1-(2-fluorophenyl)-5-hydroxy-1H-pyrazole-3-carboxylate
Reference Example 192 Ethyl 1-(2-fluorophenyl)-5-hydroxy-1H-pyrazole-3-carboxylate To a solution of 2-fluorophenylhydrazine hydrochloride (1.62 g) in ethanol (40 mL) were added potassium carbonate (2.76 g) and diethyl but-2-ynedioate (1.7 g) at room temperature, and the mixture was stirred for 4 hr with heating under reflux. The reaction mixture was allowed to cool, 1 mol/L hydrochloric acid (70 mL) and water (70 mL) were added, and the mixture was stirred for 1 hr. The precipitate was collected by filtration, washed with water, and dried under reduced pressure to give the title compound as a colorless solid (2.11 g, yield 84%). 1H-NMR (DMSO-d6) δ: 1.24-1.31 (3H, m), 4.21-4.31 (2H, m), 5.92 (1H, s), 7.32-7.40 (1H, m), 7.41-7.50 (1H, m), 7.50-7.60 (2H, m), 11.94 (1H, s).
References:
US2009/156642,2009,A1 Location in patent:Page/Page column 67