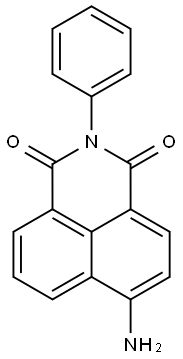
1H-benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-phenyl- synthesis
- Product Name:1H-benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-phenyl-
- CAS Number:10495-37-1
- Molecular formula:C18H12N2O2
- Molecular Weight:288.3
![6-bromo-2-phenylbenzo[de]isoquinoline-1,3-dione](/CAS/20180720/GIF/84216-52-4.gif)
84216-52-4
5 suppliers
inquiry
![1H-benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-phenyl-](/CAS/GIF/10495-37-1.gif)
10495-37-1
8 suppliers
inquiry
Yield:10495-37-1 95 %
Reaction Conditions:
Stage #1: 6-bromo-2-phenyl-1H-benzo[de]isoquinoline-1,3(2H)-dionewith 1,1-Diphenylethylen;palladium diacetate;caesium carbonate;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in 1,4-dioxane at 80;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 20;
Steps:
17 Embodiment seventeen:
Add 1g (1.0 mmol, 352.2 mg), 2 (1.2 mmol, 0.20 mL), palladium acetate (0.2 mmol, 45.0 mg), BINAP (0.2 mmol, 124.5 mg), cesium carbonate (1.5 mmol, 489.7 mg), dioxane (10 mL).Stir at 80 °C for 16 h.After the reaction was completed, the organic solvent was removed with a rotary evaporator at 50°C, tetrahydrofuran (10 mL) was added to dissolve, and aqueous hydrochloric acid (2 M, 10 mL) was added and stirred with a magnetic stirrer at 800 r/min for 30 min.After the reaction, neutralize with saturated aqueous sodium bicarbonate to pH=7, then extract with 50 mL of dichloromethane, collect the organic phase, dry, and finally layer with a mixed solvent of ethyl acetate and petroleum ether 1/2 After column purification, 3 g of yellow powder was obtained with a yield of 95%.
References:
CN115521256,2022,A Location in patent:Paragraph 0036
![6-nitro-2-phenyl-1H-benzo[de]isoquinoline-1,3(2H)-dione](/CAS/20180629/GIF/42340-35-2.gif)
42340-35-2
3 suppliers
inquiry
![1H-benz[de]isoquinoline-1,3(2H)-dione, 6-amino-2-phenyl-](/CAS/GIF/10495-37-1.gif)
10495-37-1
8 suppliers
inquiry