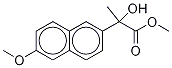
α-Hydroxy-6-Methoxy-α-Methyl-2-naphthaleneacetic Acid Methyl Ester synthesis
- Product Name:α-Hydroxy-6-Methoxy-α-Methyl-2-naphthaleneacetic Acid Methyl Ester
- CAS Number:105937-62-0
- Molecular formula:C15H16O4
- Molecular Weight:260.2851
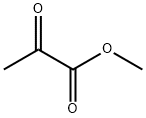
600-22-6
364 suppliers
$6.00/5g
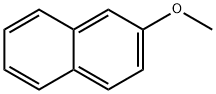
93-04-9
449 suppliers
$13.00/25g
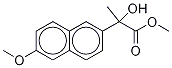
105937-62-0
10 suppliers
$165.00/1g
Yield:-
Reaction Conditions:
aluminium chloride in dichloromethane;water;
Steps:
2 Example 2
Example 2 Dichloromethane (20 mL) and 2-methoxynaphthalene (1,28 g, 8,09 mmol) were charged to a 3-necked round bottom flask equipped with a thermometer and stirrer and cooled to between 0°-5° C. Aluminium chloride (1,62 g, 12,2 mmol) was added to the reaction mixture, maintaining the temperature below 10° C. Methyl pyruvate (0,83 g, 8,09 mmol) in dichloromethane (10 mL) was added over one hour maintaining the temperature below 10° C. After addition the reaction was stirred at 5° C. for 6 hours. The reaction mixture was added to a water/ice mixture (20 mL) and the layers separated. The organic layer was concentrated to generate crude (R,S)-methyl 2-hydroxy-2-(6-methoxy-2-naphthyl)propionate. The crude was dissolved in acetone and the insoluble matter removed. After removal of solvent, the remainder was recrystallized from diisopropyl ether to give (R,S)-methyl 2-hydroxy-2-(6-methoxy-2-naphthyl)propionate (1,26 g, 4,85 mmol, 93% pure). It is to be noted that the results of this example have been subsequently proven to be overquantified, due to non-separation of isomers, by about 20-25%.
References:
US5750764,1998,A
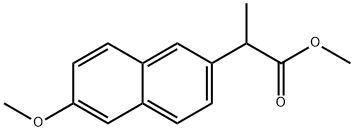
30012-51-2
19 suppliers
inquiry
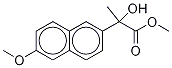
105937-62-0
10 suppliers
$165.00/1g

600-22-6
364 suppliers
$6.00/5g
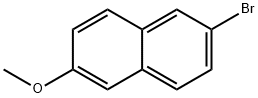
5111-65-9
328 suppliers
$6.00/5g
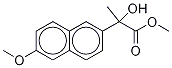
105937-62-0
10 suppliers
$165.00/1g
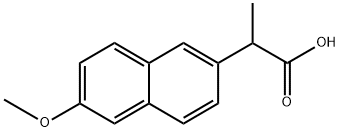
23981-80-8
159 suppliers
$8.00/1g
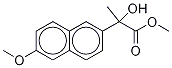
105937-62-0
10 suppliers
$165.00/1g