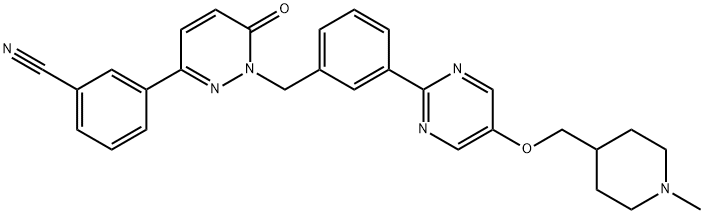
Tepotinib synthesis
- Product Name:Tepotinib
- CAS Number:1100598-32-0
- Molecular formula:C29H28N6O2
- Molecular Weight:492.57
Acetonitrile (700 ml) was added to the reaction vessel, and the compound represented by formula 7a (37.63 g, 0.1 mol), the compound represented by formula 8 (23.66 g, 0.12 mol), and potassium carbonate (34.55 g, 0.25 mol) were added under stirring.and tetrabutylammonium bromide (1.93g, 0.006mol), the reaction system was stirred and heated to reflux, and the reaction was stirred at reflux for 12h. After the reaction was completed, the temperature was cooled to room temperature, the reaction solution was concentrated under reduced pressure to dryness, and ethanol (360ml) was added. ), stir to dissolve, slowly add 1.5 mol/l hydrochloric acid aqueous solution dropwise, adjust the pH value to 1-2, cool down to 5-7 °C, stir and crystallize for 6 h, filter, and vacuum dry the solid at 45 °C for 6 h to obtain formula 1. The compound (44.42 g) was shown in a yield of 81.2% and a purity of 99.7% by HPLC.
![BenzeneMethanol, 3-[5-[(1-Methyl-4-piperidinyl)Methoxy]-2-pyriMidinyl]-](/CAS/GIF/1100598-48-8.gif)
1100598-48-8
43 suppliers
$310.00/100mg
52240-08-1
71 suppliers
$18.60/250mg

1100598-32-0
155 suppliers
$35.00/1mg
Yield:-
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 20;Cooling with ice;
Steps:
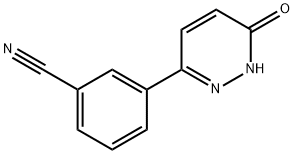
3-(1-{3-[5-(1-methyl-piperidin-4-ylmethoxy)-pyrimidin-2-yl]-benzyl}-6-oxo-1 ,6- dihydro-pyridazin-3-yl)-benzonitrile (free base) can be synthesized as described in PCT/EP2008/003473, example 40, and PCT/EP2008/005508, example 3, as follows: To a suspension of 13.0 g (56.5 mmol) of 3-(5-hydroxy-pyrimidin-2-yl)-benzoic acid methylester and 13.4 g (62.1 mmol) of N-Boc-piperidinemethanol in 115 ml THF 17.7 g (67.8 mmol) of triphenyl-phosphine are given. The suspension is cooled down to 5° C. To the suspension kept at this temperature 13.3 ml (67.8 mmol) of diisopropylazodicar- boxylate are given dropwise under stirring within 45 minutes. The reaction mixture is stirred at roomtemperature for one hour. Subsequently, further 22.2 g (84.7 mmol) of triphenylphosphine and 16.6 ml (84.7 mmol) of diisopropylazodicarboxylate are added. The reaction mixture is stirred at room temperature for 18 hours and concentrated in vacuo. The resulting solid of 4-[2-(3-methoxycarbonyl-phenyl)-pyrimidin-5-yloxymethyl]- piperidine-1 -carbonic acid tert.-butylester is sucked off, washed with diethylether and subjected to chromatography (silica gel column and dichloromethan/methanol as elu- ent/mobile phase). To a suspension of 1.71 g (3.99 mmol) of 4-[2-(3-methoxycarbonyl-phenyl)- pyrimidin-5-yloxymethyl]-piperidine-1 -carbonic acid tert.-butylester in 20 ml THF 25 ml (25 mmol) of a 1 M solution of diisobutylaluminiumhydride in THF are given dropwise under nitrogen. The reaction mixture is stirred for one hour at room temperature and mixed with a saturated solution of sodium sulfate. The resulting precipitate is sucked off and washed with THF and hot 2-propanol. The filtrate is concentrated and re- crystallized from tert.-butylmethylether, resulting in {3-[5-(1-methyl-piperidin-4- ylmethoxy)-pyrimidin-2-yl]-phenyl}-methanol as beige crystals. To a solution of 313 mg (1.00 mmol) of {3-[5-(1-methyl-piperidin-4-ylmethoxy)- pyrimidin-2-yl]-phenyl}-methanol in 2 ml THF 264 mg (1.30 mmol) of 3-(6-oxo-1 ,6- dihydro-pyridazin-3-yl)-benzonitrile and 397 mg (1.5 mmol) triphenylphosphine are added subsequently. The reaction mixture is cooled in an ice bath and 294 μl (1.5 mmol) of diisopropylazodicarboxylate are added dropwise. The reaction mixture is stirred at room temperature for 18 hours and then concentrated. The residue is subjected to chromatography (silica gel column and dichloromethan/methanol as elu- ent/mobile phase). The product containing fractions are pooled, concentrated and the residue of 3-(1-{3-[5-(1-Methyl-piperidin-4-ylmethoxy)-pyrimidin-2-yl]-benzyl}-6-oxo-1 ,6- dihydro-pyridazin-3-yl)-benzonitrile is decocted with tert.-butylmethylether, sucked off and dried in vacuo.
References:
MERCK PATENT GMBH;BECKER, Axel;KUEHN, Clemens;SAAL, Christoph;SCHADT, Oliver;DORSCH, Dieter;BOKEL, Heinz-Hermann;STIEBER, Frank;DONINI, Christina WO2010/78897, 2010, A1 Location in patent:Page/Page column 16

50-00-0
842 suppliers
$10.00/25g

1103506-80-4
2 suppliers
inquiry

1100598-32-0
155 suppliers
$35.00/1mg