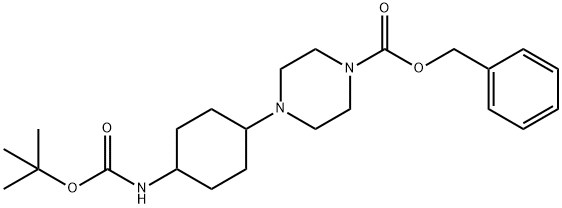
4-(4-tert-ButoxycarbonylaMino-cyclohexyl)-piperazine-1-carboxylic acid benzyl ester synthesis
- Product Name:4-(4-tert-ButoxycarbonylaMino-cyclohexyl)-piperazine-1-carboxylic acid benzyl ester
- CAS Number:1248730-88-2
- Molecular formula:C23H35N3O4
- Molecular Weight:417.54
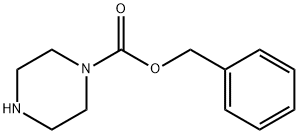
31166-44-6
213 suppliers
$5.00/1g
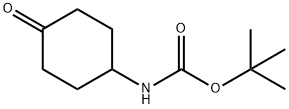
179321-49-4
306 suppliers
$5.00/1g

1248730-88-2
16 suppliers
$325.00/1g
Yield:1248730-88-2 66.4%
Reaction Conditions:
Stage #1: phenylmethyl 1-piperazinecarboxylate;N-(tert-butoxycarbonyl)-4-aminocyclohexanonewith acetic acid in methanol; for 0.5 h;
Stage #2: with 2-picoline borane complex in methanol; for 12 h;
Steps:
1
To a solution of tert-butyl N-(4-oxocyclohexyl)carbamate (10 g, 46.89 mmol, 1 eq) in methanol (150 mL) was added acetic acid (15 mL) and benzyl piperazine-1-carboxylate (10.33 g, 46.89 mmol, 1 eq). The mixture was stirred at 30° C. for 0.5 hour. Then 2-methylpyridine borane complex (10.03 g, 93.78 mmol, 2 eq) was added and the mixture was stirred at 30° C. for 12 hours. LCMS analysis of an aliquot indicated completion of the reaction. The reaction mixture was diluted with water (500 mL) and extracted with ethyl acetate (200 mL×3). The combined organic phase was washed with a saturated aqueous solution of sodium bicarbonate (200 mL) and saturated brine (300 mL×2), dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The residue was triturated with petroleum ether:ethyl acetate (V/V=5:1, 150 mL) to give benzyl 4-[4-(tert-butoxycarbonylamino)cyclohexyl]piperazine-1-carboxylate (13 g, 31.1 mmol, 66.4% yield) obtained as a white solid. MS (ESI) m/z: 418.2[M+H]+.
References:
US2019/151295,2019,A1 Location in patent:Paragraph 1484-1485