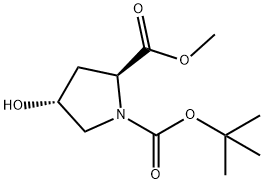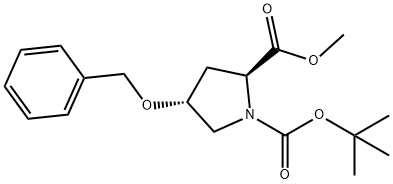
(2S,4R)-1-BOC-4-BENZYLOXY-PYRROLIDINE-2-DICARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:(2S,4R)-1-BOC-4-BENZYLOXY-PYRROLIDINE-2-DICARBOXYLIC ACID METHYL ESTER
- CAS Number:136024-60-7
- Molecular formula:C18H25NO5
- Molecular Weight:335.39
Yield:136024-60-7 97%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 16 h;Inert atmosphere;
Steps:
180.1 Step 1: 1-(Tert-butyl) 2-methyl (2S,4R)-4-(benzyloxy)pyrrolidine-1,2-dicarboxylate
To a stirred solution of 1-(tert-butyl) 2-methyl (2S,4R)-4-hydroxypyrrolidine-1,2- dicarboxylate (30.00 g, 0.12 mol) and BnBr (23.01 g, 0.13 mol) in DMF (300.00 mL) was added NaH (60% in minerial oil) (6.36 g, 0.16 mol) in portions at 0 °C under nitrogen atmosphere. The reaction mixture was stirred for 16 h at room temperature under nitrogen atmosphere. The resulting mixture was diluted with water (100 mL) at 0 °C and extracted with EA (3 x 300 mL). The combined organic layers was washed with water (2 x 100 mL), dried over anhydrous Na2SO4. After filtration, the filtrate was concentrated under reduced pressure to afford 1-(tert-butyl) 2-methyl (2S,4R)-4-(benzyloxy)pyrrolidine-1,2-dicarboxylate (40.00 g, 97%) as a yellow oil. MS ESI calculated for C18H25NO5 [M + H]+, 336.17, found, 336.20
References:
WO2021/247969,2021,A1 Location in patent:Paragraph 001166-001166
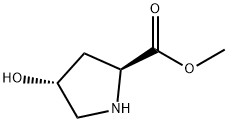
1499-56-5
40 suppliers
$40.00/100mg

136024-60-7
8 suppliers
inquiry
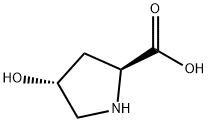
51-35-4
820 suppliers
$6.00/5g

136024-60-7
8 suppliers
inquiry

24424-99-5
815 suppliers
$13.50/25G

136024-60-7
8 suppliers
inquiry
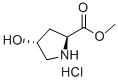
40216-83-9
309 suppliers
$6.00/5g

136024-60-7
8 suppliers
inquiry