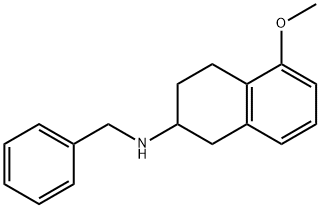
5-methoxy-1,2,3,4-tetrahydro-N-(phenylmethyl)- 2-Naphthalenamine (Rotigotine) synthesis
- Product Name:5-methoxy-1,2,3,4-tetrahydro-N-(phenylmethyl)- 2-Naphthalenamine (Rotigotine)
- CAS Number:136247-07-9
- Molecular formula:C18H21NO
- Molecular Weight:267.37
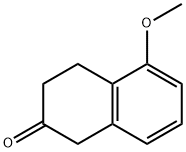
32940-15-1
304 suppliers
$13.43/250mgs:
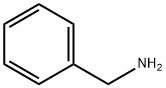
100-46-9
461 suppliers
$5.00/5G

136247-07-9
21 suppliers
inquiry
Yield:136247-07-9 74%
Reaction Conditions:
Stage #1: 5-methoxy-2-tetralone;benzylaminewith acetic acid in dichloromethane at 20; for 4 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 0 - 20; for 15.5 h;
Stage #3: with water;sodium hydrogencarbonate in dichloromethane at 0; pH=8.0; for 0.25 h;
Steps:
A
Example A N-Benzyl-N-(5-methoxy-1,2,3,4-tetrahydronaphthalen-2-yl)amine To a solution of 5-methoxy-2-tetralone (30 g, 170.24 mmol) dissolved in CH2Cl2 (250 mL) were added benzylamine (23 mL, 212.80 mmol) and AcOH (0.97 mL, 17.02 mmol), and the mixture was stirred for 4 h at room temperature. It was then cooled to 0 °C and NaB(OAc)3H (0.38 eq, 13.71 g, 64.69 mmol) was added over a period of 20 min. After 1 h stirring at 0 °C, NaB(OAc)3H (1.07 eq, 38.61 g, 182.16 mmol) was added over a period of 30 min. It was added CH2Cl2 (100 mL), the reaction mixture warmed to room temperature and stirred for 15 h. The mixture was cooled again to 0°C, and H2O (200 mL) was added slowly. The pH of the solution was adjusted to 8.0 by adding NaHCO3 saturated aqueous solution (300 mL), and the mixture was stirred at 0 °C for 15 min. The layers were separated, and the aqueous phase was extracted with CH2Cl2 (2x150 mL). All organic phases were combined, dried over anhydrous Na2SO4 and concentrated in vacuo. The residue (58.7 g) was purified by flash chromatography on silica gel (40:60:1-100:0:1 AcOEt/Hexane/Et3N), followed by trituration with hexane, affording 33.87 g of the title compound (Rf= 0.5 (10% MeOH/CH2Cl2), yellow solid, 74% yield). 1H-NMR (CDCl3, 250 MHz, δ): 7.26-7.12 (m, 5H, ArH); 7.00 (dd, J = 8.0 y 7.7 Hz, 1H, ArH); 6.60 (m, 2H, ArH); 3.82 (s, 2H, CH2); 3.72 (s, 3H, CH3); 2.88 (m, 2H, CH2); 2.51 (m, 2H, CH2); 1.99 (m, 1H, CH); 1.48 (m, 2H, CH2).
References:
EP1956006,2008,A1 Location in patent:Page/Page column 26

32940-15-1
304 suppliers
$13.43/250mgs:

136247-07-9
21 suppliers
inquiry
575-44-0
347 suppliers
$7.00/10g

136247-07-9
21 suppliers
inquiry
3900-49-0
194 suppliers
$10.00/1g

136247-07-9
21 suppliers
inquiry