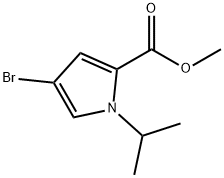
1H-Pyrrole-2-carboxylic acid, 4-bromo-1-(1-methylethyl)-, methyl ester synthesis
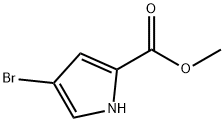
- Product Name:1H-Pyrrole-2-carboxylic acid, 4-bromo-1-(1-methylethyl)-, methyl ester
- CAS Number:1367874-83-6
- Molecular formula:C9H12BrNO2
- Molecular Weight:246.1
Yield:1367874-83-6 84%
Reaction Conditions:
Stage #1: methyl 4-bromo-1H-pyrrole-2-carboxylatewith sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
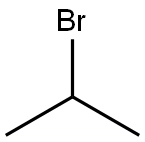
Stage #2: isopropyl bromide in N,N-dimethyl-formamide at 90;
Steps:
Intermediate 46Step 1Methyl 4-bromo-1 -isopropyl-1 H-pyrrole-2-carboxylateTo a mixture of methyl 4-bromo-1/-/-pyrrole-2-carboxylate (4.5 g, 0.022 mol) in DMF (20 ml_) was added NaH (60% dispersion, 2.6 g, 0.066 mol) at 0 °C. The reaction was stirred at 0 °C for 30 minutes. 2-Bromopropane (8.1 g, 0.066 mol) was added to the reaction mixture and stirred overnight at 90 °C. After cooling to room temperature, the reaction was quenched with 100 mL of water and extracted with ethyl acetate (3 x 50 mL). The organic layer was washed with water and brine, dried over anhydrous Na2S04, filtered and concentrated. The residue was purified by column chromatography (50% ethyl acetate in pet. ether). The desired compound was obtained as a yellowish oil (4.55 g, 84%). LCMS (Method A): m/z = 246 [M+Hf; 2.19 min
References:
WO2012/38743,2012,A1 Location in patent:Page/Page column 106