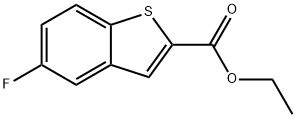
Benzo[b]thiophene-2-carboxylic acid, 5-fluoro-, ethyl ester synthesis
- Product Name:Benzo[b]thiophene-2-carboxylic acid, 5-fluoro-, ethyl ester
- CAS Number:13771-69-2
- Molecular formula:C11H9FO2S
- Molecular Weight:224.25
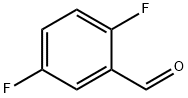
2646-90-4
276 suppliers
$6.00/1g
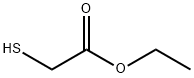
623-51-8
231 suppliers
$7.00/25g
![Benzo[b]thiophene-2-carboxylic acid, 5-fluoro-, ethyl ester](/CAS/20180629/GIF/13771-69-2.gif)
13771-69-2
15 suppliers
$31.19/250mg
Yield:13771-69-2 60.5%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20 - 60; for 12.5 h;Cooling with ice;
Steps:
Ethyl 5-fluorobenzo[b]thiophene-2-carboxylate (8)[0131] To a mixture of 2,5-difluorobenzaldehyde (217.28 μ, 2 mmol) and potassium carbonate (595.53 mg, 2.5 mmol) in DMF (3 mL) was added ethyl thioglycolate (219.30 μ, 2 mmol) dropwise with ice cooling.7 The mixture was stirred at room temperature for 30 min and at 60 °C for 12 h, poured into water, and extracted with EtOAc. The extract was washed with water, dried, and concentrated, and the residue was suspended in EtOH and collected by filtration gave 8 as crystals; (271.34 mg, 60.5%). 1H-NMR (300 MHz, CDC13): δ 8.00 (s, 1H), 7.83-7.78 (m, 1H), 7.55-7.51 (m, 1H), 7.26-7.19 (m, 1H), 4.46-4.39 (q, 2H), 1.46-1.41 (t, 3H). 13C NMR (300 MHz, CDC13): δ 162.47, 159.25, 139.63, 137.60, 136.29, 129.75, 124.11, 116.24, 110.65, 61.74, 14.29.
References:
WO2012/99785,2012,A2 Location in patent:Page/Page column 34-35