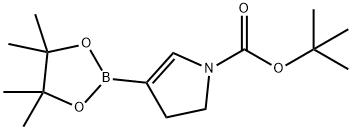
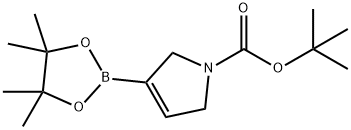
1-BOC-2,3-Dihydropyrrole-4-boronic acid, pinacol ester synthesis
- Product Name:1-BOC-2,3-Dihydropyrrole-4-boronic acid, pinacol ester
- CAS Number:1401165-14-7
- Molecular formula:C15H26BNO4
- Molecular Weight:295.18
101385-93-7
471 suppliers
$5.00/1g

73183-34-3
559 suppliers
$6.00/5g
212127-83-8
147 suppliers
$50.00/250mg

1401165-14-7
26 suppliers
$45.00/25mg
Yield:-
Reaction Conditions:
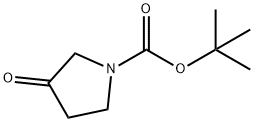
Stage #1:3-oxo-pyrrolidine-1-carboxylic acid tert-butyl ester with lithium hexamethyldisilazane in tetrahydrofuran at -78; for 0.333333 h;
Stage #2: with N,N-phenylbistrifluoromethane-sulfonimide in tetrahydrofuran at 20; for 1 h;
Stage #3:bis(pinacol)diborane with 1,1'-bis-(diphenylphosphino)ferrocene;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane at 80;Overall yield = 86 %; Overall yield = 800 g;
Steps:
12.B Step B. tert-butyl 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,3-dihydro-lH-pyrrole- 1-carboxylate and tert-butyl 3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,5-dihydro- lH-pyrrole-l-carboxylate
Step B. tert-butyl 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,3-dihydro-lH-pyrrole- 1-carboxylate and tert-butyl 3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,5-dihydro- lH-pyrrole-l-carboxylate A mixture of tert-butyl 4-(((trifluoromethyl)sulfonyl)oxy)-2,3-dihydro-lH-pyrrole-l- carboxylate and tert-butyl 3-(((trifluoromethyl)sulfonyl)oxy)-2,5-dihydro-lH-pyrrole-l- carboxylate (1 g, 3.15 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(l,3,2-dioxaborolane) (880 mg, 3.47 mmol), potassium acetate (927 mg, 9.46 mmol), Pd(dppf)2Cl2 (100 mg), and dppf (75 mg) in 1,4-dioxane (10 mL) was stirred at 80 °C overnight. The reaction mixture was concentrated under reduced pressure. The residue was partitioned with water (10 mL) and EtOAc (20 mL). The organic layer was separated, washed with brine (10 mL), dried over magnesium sulfate, filtered and concentrated under reduced pressure. The residue was purified by silica gel chromatography using petroleum ether as eluting solvent to afford a mixture of tert-butyl 4-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,3-dihydro-lH- pyrrole-1 -carboxylate and tert-butyl 3-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)-2,5- dihydro-lH-pyrrole-1 -carboxylate as a white solid (800 mg, 86%). LCMS (ESI) m/z: 240.2 [M-56+H]+.
References:
BEAUFOUR-IPSEN (TIANJIN) PHARMACEUTICAL CO., LTD.;AUVIN, Serge;LANCO, Christophe;DUTRUEL, Oliver;CHAO, Qi;GU, Kaichun WO2015/100617, 2015, A1 Location in patent:Page/Page column 50

101385-93-7
471 suppliers
$5.00/1g

1401165-14-7
26 suppliers
$45.00/25mg