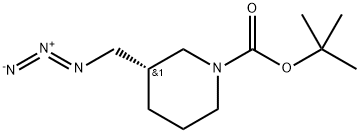
(S)-Tert-Butyl 3-(azidomethyl)piperidine-1-carboxylate synthesis
- Product Name:(S)-Tert-Butyl 3-(azidomethyl)piperidine-1-carboxylate
- CAS Number:140645-22-3
- Molecular formula:C11H20N4O2
- Molecular Weight:240.31
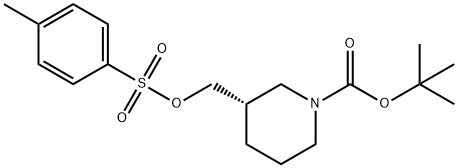
191092-08-7
1 suppliers
inquiry

140645-22-3
3 suppliers
inquiry
Yield:140645-22-3 92%
Reaction Conditions:
with sodium azide;sodium iodide in N,N-dimethyl-formamide at 70; for 4 h;
Steps:
(S)-tert-butyl3-(azidomethyl)piperidine- 1 -carboxylate
(S)-tert-butyl3-(azidomethyl)piperidine- 1 -carboxylate._(S)-tert-butyl 3-((tosyloxy)methyl)piperidine-1 - carboxylate (8.85 g, 24 mmol) was dissolved in DMF (100 ml), and then NaN3 (6.24 g, 96 mmol) and Nal (0.36 g, 2.40 mmol) were added, followed by stirring at 70° C. for 4 hours. After the completion of the reaction, the reaction mixture was extracted with H20, EA, and brine, followed by drying (Na2SO4), filtration, and concentration under reduced pressure, and the residue was purified by column chromatography (EA:Hex=1:10), to give (S)-tert-butyl 3-(azidomethyl)piperi- dine-i-carboxylate (5.38 g, 92%).‘H-NMR (300 MHz, CDC13) ? 4.09-3.82 (m, 2H),3.27-3.16 (m, 2H), 2.95-2.54 (m, 2H), 1.88-1.62 (m, 3H),1.52-1.42 (m, 1H), 1.47 (s, 9H), 1.30-1.20 (m, 1H)
References:
US2015/259350,2015,A1 Location in patent:Paragraph 0235; 0236
![(S)-[[(Methylsulfonyl)oxy]methyl]piperidine-1-carboxylic Acid tert-Butyl Ester](/CAS/20200401/GIF/364356-44-5.gif)
364356-44-5
4 suppliers
inquiry

140645-22-3
3 suppliers
inquiry
140695-84-7
174 suppliers
$7.00/250mg

140645-22-3
3 suppliers
inquiry

24424-99-5
817 suppliers
$13.50/25G

140645-22-3
3 suppliers
inquiry
37675-18-6
252 suppliers
$5.00/1g

140645-22-3
3 suppliers
inquiry