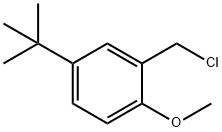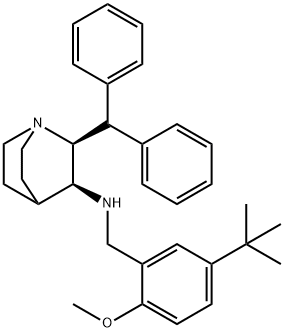
(2S,3S)-2-Benzhydryl-N-(5-tert-butyl-2-Methoxybenzyl)quinuclidin-3-aMine synthesis
- Product Name:(2S,3S)-2-Benzhydryl-N-(5-tert-butyl-2-Methoxybenzyl)quinuclidin-3-aMine
- CAS Number:147116-67-4
- Molecular formula:C32H40N2O
- Molecular Weight:468.67
Yield:147116-67-4 85.3%
Reaction Conditions:
with dmap;triethylamine in N,N-dimethyl-formamide at 0 - 30;
Steps:
5 Embodiment 5: marrow Tanzania free alkali preparation
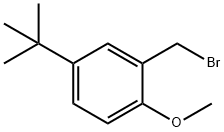
The reaction bottle point pen V compound (15 g, 51.3 mmol, 1.0 eq.) and DMF (100 ml), stirring to dissolve in the system add triethylamine (26 g, 257 mmol, 5.0 eq.) and of a catalytic amount of DMAP (10 mg), after the adding of system cooling to 0 °C, then to a reaction system slow instillment type compound VI (X=Br, 17.15 g, 1.3 eq.) of DMF (50 ml) solution. After dropping, the system slowly natural heating up to 30 °C stirring reaction to the TLC tracking the starting material disappeared. System natural cooling to room temperature, high vacuum pressure system for removing organic solvent, residue added CH2Cl2 (200 ml) and H2O (100 ml), separating the organic phase, the aqueous phase CH2Cl2 extraction (3 × 50 ml). The combined organic phase, the organic the trunk and salt is washed with water (100 ml), anhydrous Na2SO4 desolution of the after drying under reduced pressure, the residue with ethanol (75 ml), heated to reflux stirring dissolves clear, the temperature slowly drops to 0 °C heat preservation after stirring 12 hours, filtering, the resulting solid 40 °C obtained by vacuum drying the marrow Tanzania free alkali (20.5 g, 85.3%).
References:
CN106977512,2017,A Location in patent:Paragraph 0027; 0028
![Bicyclo[2.2.1]heptane-1-methanesulfonic acid, 7,7-dimethyl-2-oxo-, (1R,4S)-, compd. with (2S,3S)-N-[[5-(1,1-dimethylethyl)-2-methoxyphenyl]methyl]-2-(diphenylmethyl)-1-azabicyclo[2.2.2]octan-3-amine (1:1) (9CI)](/CAS/20211123/GIF/862543-52-0.gif)
862543-52-0
1 suppliers
inquiry

147116-67-4
83 suppliers
inquiry
![(2S)-2-benzhydryl-1-azabicyclo[2.2.2]octan-3-one](/CAS/20180601/GIF/683206-53-3.gif)
683206-53-3
1 suppliers
inquiry

147116-67-4
83 suppliers
inquiry
![1-Azabicyclo[2.2.2]octan-3-amine, 2-(diphenylmethyl)-, (2S,3S)-](/CAS/20200611/GIF/142035-23-2.gif)