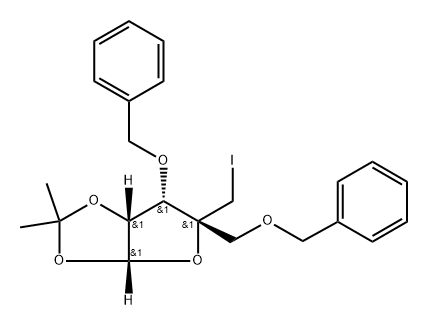
4-C-(Iodomethyl)-1,2-di-O-(1-methylethylidene)-3,5-bis-O-(phenylmethyl)--D-ribofuranose synthesis
- Product Name:4-C-(Iodomethyl)-1,2-di-O-(1-methylethylidene)-3,5-bis-O-(phenylmethyl)--D-ribofuranose
- CAS Number:153186-14-2
- Molecular formula:C23H27IO5
- Molecular Weight:510.37
![1,2-O-(1-methylethylidene)-4-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-L-Lyxofuranose](/CAS/20180808/GIF/153186-10-8.gif)
153186-10-8
51 suppliers
inquiry

153186-14-2
4 suppliers
inquiry
Yield:-
Reaction Conditions:
with 1H-imidazole;iodine;triphenylphosphine in 1,4-dioxane;benzene at 85; for 60 h;
Steps:
1.H Step H
Step H 1,2-O-isopropylidene-3,5-di-O-benzyl-4-C-iodomethyl-α-D-ribofuranose Iodine (14.2 g, 55.83 mmol) was added to a solution of 1,2-O-isopropylidene-3,5-di-O-benzyl-4-hydroxymethyl-α-D-ribofuranose (11.51 g, 28.78 mmol), triphenylphosphine (29.17 g, 111.22 mmol), and imidazole (7.56 g, 111.06 mmol) in a mixture of dry benzene (120 mL) and dry 1,4-dioxane (30 mL). The reaction mixture was stirred at 85° C. under N2 atmosphere for 24 h. Additional portions of triphenylphosphine (9.72 g), imidazole (2.52 g) and iodine (4.73 g) were added and the stirring was continued for total of 36 h. The reaction mixture was concentrated into a crude residue which was poured into 10% Na2S2O3 solution and extracted with ethyl acetate. The organic layer was washed with saturated aqueous NaHCO3 solution, saturated brine solution, dried (MgSO4) and concentrated to a crude residue, which was applied to a column of silica gel eluted with hexane-ethyl acetate (4:1) to give the title compound as a syrup. 1H NMR (CD2Cl2, 200 MHz): δ 7.35-7.32 (m, 5H, ArH), 5.72 (d, 1H, J=3.8Hz, H-1), 4.75-4.54 (m, 5H, H-2, 2 OCH2Ph), 4.28 (d, 1H, J=5.0Hz, H-3), 3.93 (d, 1H, Jgem=11.2 Hz, CHI), 3.55 (dd, 2H, CH2OBn), 3.49 (d, 1H, Jgem=11.2Hz, CHI), 1.59 (s, 3H, CH3), 1.33 (s, 3H, CH3). 13C NMR (CD2Cl2, 50 MHz): δ 138.32, 137.92, 128.56, 128.53, 128.08, 128.00, 127.96, 127.87, 113.67, 109.99, 103.78, 83.93, 79.34, 74.70, 73.80, 72.79, 26.89, 25.96, 11.34.
References:
US2004/229840,2004,A1 Location in patent:Page 12
63593-03-3
149 suppliers
$38.00/250mg

153186-14-2
4 suppliers
inquiry