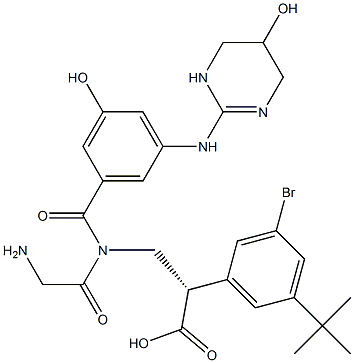
(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine synthesis
- Product Name:(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine
- CAS Number:1564286-55-0
- Molecular formula:C26H32BrN5O6
- Molecular Weight:590.47
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine](/CAS/20150408/GIF/1564286-55-0.gif)
1564286-55-0
63 suppliers
$29.00/1mg
Yield: 2.244 g
Reaction Conditions:
with lithium hydroxide monohydrate in water;acetonitrile at 20; for 1.5 h;
Steps:
1.5 Preparation of (3S)-N-[3-hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyrimidinyl)amino]benzoyl]glycyl-3-(3-bromo-5-tert-butylphenyl)-β-alanine (Example 1)
A mixture of 2-(3-hydroxy-5-((5-hydroxy-1,4,5,6-tetrahydropyrimidin-2-yl)amino)benzamido)acetic acid (Example B) (1.92 g, 6.23 mmol), and (S)-ethyl 3-amino-3-(3-bromo-5-tert-butylphenyl)propionate hydrochloride (2.25 g, 6.17 mmol) was dissolved in DMF (20.0 mL) and dichloromethane (10.0 mL) to give a cream suspension. 1-hydroxybenzatriazole hydrate was added to above reaction mixture and the reaction mixture was stirred in an ice-bath under nitrogen atmosphere for 10 min. N,N′-diisopropylcarbodiimide (DIC) was added and the reaction mixture was stirred at room temperature overnight under nitrogen atmosphere. The solvent was evaporated in vacuo to give a pale yellow viscous gummy residue. The residue was dissolved in acetonitrile (50 mL) and filtered to remove precipitated urea. Evaporation of the filtrate in-vacuo afforded a pale yellow to cream foamy residue of the crude (3S)-N-[3-hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyrimidinyl)imino]benzoyl]glycyl-3-(3-bromo-5-tert-butylphenyl)-β-alanine ethyl ester. To a suspension of the crude ester in a 1:1 mixture of water/acetonitrile (14.0 mL) was added lithium hydroxide monohydrate (2.07 g, 49.38 mmol) at room temperature and the reaction mixture was stirred at room temperature for 1.5 h. The reaction mixture was acidified with TFA and evaporated in vacuo to give a cream foamy solid. The solid was purified by reverse-phase preparative HPLC (10-90% water/acetonitrile containing 0.05% TFA) to afford the desired product, after lyophilization, as a colorless powder (2.244 g). LC/MS shows the desired product's mass: m/z 590 (79BrM+H) and 592 (81BrM+H); Calcd for C26H32BrN5O6: 590.47. 1H NMR (400 MHz, DMSO-d6): δ 1.27 (s, 9H, (CH3)3C-), 2.70 (d, J=7.31 Hz, 2H, -CH2-COOH), 3.16 (dd, J=12.22, 3.10 Hz, 2H), 3.33 (brd, J=12.44 Hz, 2H), 3.87 (d, J=5.81 Hz, 2H), 4.08 (appt/m, J=2.92 Hz, 1H), 5.18 (q, J=7.62 Hz, 1H, -NH-CH-CH2-COOH), 6.75 (t, J=2.03 Hz, 1H), 7.13 (dt, J=10.70 and 1.50 Hz, 2H), 7.34 (dt, J=9.54 and 1.50 Hz, 2H), 7.39 (appt, J=1.72 Hz, 1H), 8.09 (brs, 2H), 8.51 (d, J=8.30 Hz, 1H), 8.61 (brt, J=5.88 Hz, 1H), 9.57 (s, 1H), 10.00 (brs, 1H), 12.35 (brs, 1H, -COOH). 1H NMR spectrum of the sample was consistent with the suggested structure of the product
References:
Saint Louis University;RUMINSKI, Peter;GRIGGS, David US2014/51715, 2014, A1 Location in patent:Paragraph 0132; 0133
1541197-65-2
1 suppliers
inquiry
![(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine](/CAS/20150408/GIF/1564286-55-0.gif)
1564286-55-0
63 suppliers
$29.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine](/CAS/20150408/GIF/1564286-55-0.gif)
1564286-55-0
63 suppliers
$29.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine](/CAS/20150408/GIF/1564286-55-0.gif)
1564286-55-0
63 suppliers
$29.00/1mg

129316-09-2
137 suppliers
$10.00/1g
![(3S)-N-[3-Hydroxy-5-[(1,4,5,6-tetrahydro-5-hydroxy-2-pyriMidinyl)aMino] benzoyl]glycyl-3-(3-broMo-5-t-butylphenyl)-beta-alanine](/CAS/20150408/GIF/1564286-55-0.gif)
1564286-55-0
63 suppliers
$29.00/1mg