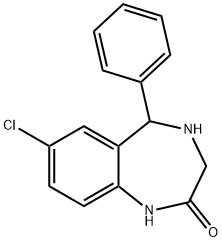
7-Chloro-1,3,4,5-tetrahydro-5-phenyl-2H-1,4-benzodiazepin-2-one synthesis
- Product Name:7-Chloro-1,3,4,5-tetrahydro-5-phenyl-2H-1,4-benzodiazepin-2-one
- CAS Number:1824-69-7
- Molecular formula:C15H13ClN2O
- Molecular Weight:272.73
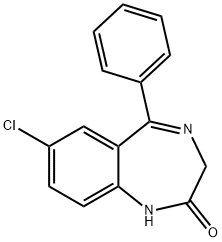
1088-11-5
2 suppliers
$18.00/1mg

1824-69-7
4 suppliers
inquiry
Yield:1824-69-7 55%
Reaction Conditions:
with acetic acid in methanol at 20; for 120 h;
Steps:
1 Preparation of 7-chloro-5-phenyl-2,3,4,5-tetrahydro-1H-1,4-benzodiazepin-2-one (compound 171)
Preparation of 7-chloro-5-phenyl-2,3,4,5-tetrahydro-1H-1,4-benzodiazepin-2-one (compound 171)
Compound 170 (1 eq., 0.22 mmol, 60 mg) is dissolved in 2 ml of methanol. The reducing agent on polymer beads (3 eq., 0.66 mmol, loading: 4.4 mmol/g, 150 mg) is added, as are a few drops of acetic acid (50 μl). The reaction takes place at ambient temperature for 5 days. After filtration and then evaporation, the product obtained is purified by silica gel chromatography (cyclohexane/ethyl acetate in a gradient of from 80:20 to 1:2), and the desired product 171 is obtained with a yield of 55%.
1H NMR (400 MHz, CDCl3) δ: 9.15 (s, 1H, NH), 7.40-6.86 (m, 8H, HAr), 5.20 (s, 1H, CH), 3.45-3.36 (q, J=10-13.6 Hz, 2H, CH2), 3.10 (s, 1H, NH).
MS (ES) m/z 273 (M+H+), 221, 91.
References:
US2016/83355,2016,A1 Location in patent:Paragraph 0364; 0365; 0366; 0367; 0368
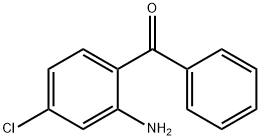
4076-50-0
22 suppliers
inquiry

1824-69-7
4 suppliers
inquiry