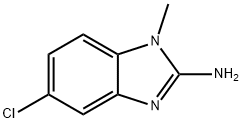
1H-Benzimidazol-2-amine,5-chloro-1-methyl-(9CI) synthesis
- Product Name:1H-Benzimidazol-2-amine,5-chloro-1-methyl-(9CI)
- CAS Number:103748-25-0
- Molecular formula:C8H8ClN3
- Molecular Weight:181.62
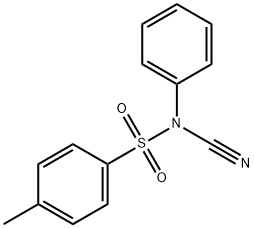
55305-43-6
65 suppliers
$23.00/100mg
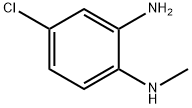
59681-66-2
56 suppliers
$52.00/100mg

103748-25-0
16 suppliers
inquiry
Yield:103748-25-0 86%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran;hexane at 5 - 20; for 1 h;Green chemistry;
Steps:
Typical Experimental Procedure for the Synthesis of 2-Aminobenzaxozole from o-Aminophenol
General procedure: To a solution of o-aminophenol (400 mg, 3.67 mmol) and NCTS (998 mg, 3.67 mmol) in THF (6 mL), 1 M LiHMDS in hexane (3.67 mL, 3.67 mmol) was added and stirred at 5 °C to r.t. for 1h. Then the reaction mixture was poured in ice water and stirred for 15 min. Then extracted with EtOAc, the organic layer was separated. The organic layer was washed with brine solution.Then organic layer was dried over Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography to obtained pure 2-aminobenzaxozole in 90% yield (471 mg).
References:
Kasthuri, Mahesh;Babu, H. Sharath;Kumar, K. Shiva;Sudhakar, Ch.;Kumar, P. V. Nagendra [Synlett,2015,vol. 26,# 7,art. no. ST-2014-B0948-L,p. 897 - 900]
![Ethanol, 2-[(4-chloro-2-nitrophenyl)methylamino]-](/CAS/20210305/GIF/103748-12-5.gif)
103748-12-5
0 suppliers
inquiry

103748-25-0
16 suppliers
inquiry
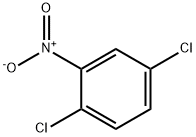
89-61-2
7 suppliers
$10.00/5g

103748-25-0
16 suppliers
inquiry

15950-17-1
64 suppliers
$43.00/100mg

103748-25-0
16 suppliers
inquiry

59681-66-2
56 suppliers
$52.00/100mg

103748-25-0
16 suppliers
inquiry