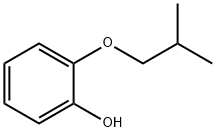
2-(2-Methylpropyloxy)phenol synthesis
- Product Name:2-(2-Methylpropyloxy)phenol
- CAS Number:21315-20-8
- Molecular formula:C10H14O2
- Molecular Weight:166.22
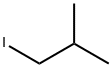
513-38-2
175 suppliers
$62.00/25mL
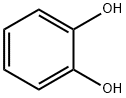
120-80-9
491 suppliers
$13.00/25g

21315-20-8
12 suppliers
$45.00/50mg
Yield:21315-20-8 80%
Reaction Conditions:
with potassium carbonate in acetone; for 72 h;Reflux;Williamson synthesis;
Steps:
4.2.1. 2-Isopropoxyphenol (1a)
General procedure: A mixture of catechol (11.0 g, 100 mmol), 2-bromopropane (9.40 mL, 100 mmol), and K2CO3 (34.5 g, 250 mmol) in acetone (100 mL) was refluxed for 1 day and the reaction progress monitored by TLC. The salts were filtered off rinsing with acetone and the filtrate concentrated. The residue was partitioned between diluted aq HCl (100 mL) and Et2O (80 mL), and the product extracted with Et2O (2×80 mL). The organic layer is dried (Na2SO4) and concentrated. Kugelrohr distillation (120 °C, 10 mbar) afforded a colorless liquid (13.4 g, 88%);
References:
Stephan, Michel;Zupancic, Borut;Mohar, Barbara [Tetrahedron,2011,vol. 67,# 34,p. 6308 - 6315] Location in patent:experimental part

81995-32-6
27 suppliers
$70.00/1g

21315-20-8
12 suppliers
$45.00/50mg
![Phenol, 2-[(tetrahydro-2H-pyran-2-yl)oxy]-](/CAS/20180529/GIF/21645-25-0.gif)
21645-25-0
1 suppliers
inquiry

21315-20-8
12 suppliers
$45.00/50mg

120-80-9
491 suppliers
$13.00/25g

21315-20-8
12 suppliers
$45.00/50mg