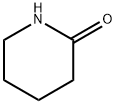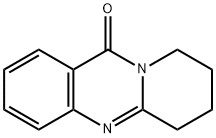
2,3-Butanoquinazoline-4(3H)-one synthesis
- Product Name:2,3-Butanoquinazoline-4(3H)-one
- CAS Number:2446-62-0
- Molecular formula:C12H12N2O
- Molecular Weight:200.24
![Benzamide, 2-[(5-chloro-1-oxopentyl)amino]-](/CAS/20210305/GIF/107954-46-1.gif)
107954-46-1
0 suppliers
inquiry

2446-62-0
18 suppliers
inquiry
Yield:2446-62-0 92%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran at 20;Inert atmosphere;
Steps:
Synthesis of 2,3-dihydropyrrolo[1,2-a]quinazolin-5(1H)-ones: general procedure B
General procedure: Toasolutionoftheappropriatesubstituted2-(4-chlorobutanamido)-benzamidessubstrate(1.0eq)inTHF(10mLpermmolsubstrate)wasaddedtBuOK(2.0eq).ThereactionwasstirredatroomtemperatureuntilTLCindicatedcompletion,thensolventwasremovedinvacuo,theresultingresiduere-dissolvedinCH2Cl2(20mL)andNaHCO3(15mL),andtheaqueouslayerextractedwithCH2Cl2(520mL).ThecombinedorganicphasesweredriedoverMgSO4andsolventremovedinvacuo.TheproductswereobtainedafterpurificationoftheresiduebyFCC.
References:
Sutherell, Charlotte L.;Ley, Steven V. [Synthesis,2017,vol. 49,# 1,art. no. SS-2016-Z0435-OP,p. 135 - 144] Location in patent:supporting information

60915-16-4
0 suppliers
inquiry

2446-62-0
18 suppliers
inquiry

328571-44-4
0 suppliers
inquiry

2446-62-0
18 suppliers
inquiry
27219-07-4
152 suppliers
$6.00/250mg
118-92-3
4 suppliers
$23.00/250g

2446-62-0
18 suppliers
inquiry