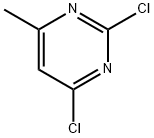
2,4-Dichloro-6-methylpyrimidine synthesis
- Product Name:2,4-Dichloro-6-methylpyrimidine
- CAS Number:5424-21-5
- Molecular formula:C5H4Cl2N2
- Molecular Weight:163
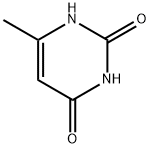
626-48-2
374 suppliers
$10.00/25 g

5424-21-5
309 suppliers
$10.00/5g
Yield:5424-21-5 90%
Reaction Conditions:
with trichlorophosphate at 90 - 100; for 10 h;Inert atmosphere;Large scale;
Steps:
9.2 Step 2
Pyrimidine 7b (1.0 kg, 7.9 mol, 1.0 eq.) and POCi:? (8.0 L, 8.0 vol.) were charged into a flask (20 L) under a 2 atmosphere. The mixture was stirred and heated to 90-100 °C. The reaction mixture turned to a clear solution after 2 h. The reaction proceeded at 90-100°C for about 8 h. The reaction was monitored by HPLC until 7b was consumed to below 0.1%. (HPLC showed 7b consumed completely; 7a was 97.0A% and impurity 7a- 1 was 2.3A%). The reaction was combined with an additional batch of the reaction solution for workup. The reaction mixture was concentrated to remove the majority of POCh. DCM (10.0 L, 10.0 vol.) was added to the residue. The resulting solution was added dropwise slowly into 25% of aq. K2CO3 at <40°C. The pH of the aqueous layer was 3-4. The organic phase was separated and the aqueous phase was extracted with DCM (10.0 L, 10.0 vol.). The combined organic phase was dried over anhydrous Na2S04 (-200 g). The mixture was filtered and the filter cake was washed with DCM (500 mL x 2), The filtrate was evaporated to dryness at 40 °C under reduced pressure. The solid was dried at 40 °C to afford yellow 7a (2.1 kg) with 99.4% HPLC purity in 90% isolated yield.
References:
EPIZYME, INC.;CAMPBELL, John Emmerson;DUNCAN, Kenneth William;MILLS, James Edward John;MUNCHHOF, Michael John WO2019/79540, 2019, A1 Location in patent:Paragraph 01612

626-48-2
374 suppliers
$10.00/25 g

5424-21-5
309 suppliers
$10.00/5g
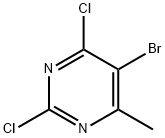
56745-01-8
80 suppliers
$14.00/1g

5424-21-5
309 suppliers
$10.00/5g
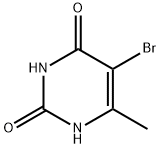
15018-56-1
109 suppliers
$26.00/250mg

5424-21-5
309 suppliers
$10.00/5g
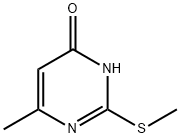
6328-58-1
92 suppliers
$10.60/1gm:

5424-21-5
309 suppliers
$10.00/5g