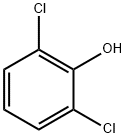
2,6-Dichlorophenol synthesis
- Product Name:2,6-Dichlorophenol
- CAS Number:87-65-0
- Molecular formula:C6H4Cl2O
- Molecular Weight:163
Yield:120-83-2 85.43% ,87-65-0 13.31%
Reaction Conditions:
with iron(III) chloride;diphenyl sulfide;boric acid at 40 - 80;
Steps:
3
In a 5,000-liter chlorination kettle with an exhaust gas treatment system, the molar ratio of o-chlorophenol phenol, catalyst diphenyl sulfide, ferric chloride and boric acid (diphenyl sulfide, ferric chloride and boric acid was 1.0 : 1.0: 1.0) Open the water pump, keep the vacuum at -0.005MPa, start to adjust the chlorine speed, the chlorination temperature is maintained at 40 ~ 80 ° C, and start the circulating pump, so that the reaction began to cycle. Until the control analysis found that trichlorophenol ≥ 0.5% can be regarded as the end of dichlorination. And then improve the vacuum to 0.09MPa (away the reaction of the remaining sulfur dioxide and hydrogen chloride), with the pH test paper to detect the tail gas is not significant acid, that is, the end of the catch; and then open the bottom of the discharge valve, the material into the crude phenol in. Gas chromatography analysis revealed 0.54% o-chlorophenol, 0.06% p-chlorophenol, 13.31% 2,6-dichlorophenol, 85.43% 2,4-dichlorophenol and 0.50% of 2,4,6-trichlorophenol.
References:
CN106349025,2017,A Location in patent:Paragraph 0019
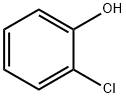
95-57-8
338 suppliers
$10.00/5g

87-65-0
402 suppliers
$10.00/10g
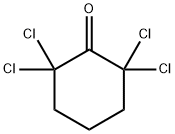
3776-30-5
28 suppliers
inquiry

87-65-0
402 suppliers
$10.00/10g

108-95-2
708 suppliers
$14.00/25g

87-65-0
402 suppliers
$10.00/10g
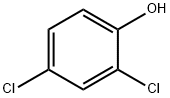
120-83-2
389 suppliers
$12.90/100g
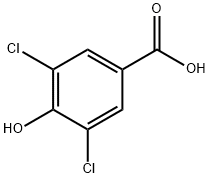
3336-41-2
163 suppliers
$9.00/1g

87-65-0
402 suppliers
$10.00/10g