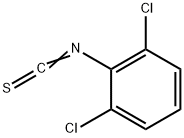
2,6-DICHLOROPHENYL ISOTHIOCYANATE synthesis
- Product Name:2,6-DICHLOROPHENYL ISOTHIOCYANATE
- CAS Number:6590-95-0
- Molecular formula:C7H3Cl2NS
- Molecular Weight:204.08
Yield: 70%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20;
Steps:
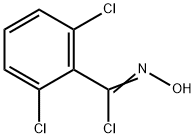
Intermediate of Example-7k: 1, 3-Dichloro-2-isothiocyanatobenzene (15)
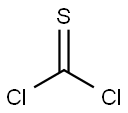
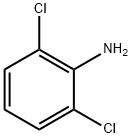
N, N-Diisopropylethylamine (1.86 g, 18.41 mmol) was added slowly to a stirred solution of 2, 6-dichloroaniline (2 g, 12.34 mmol) in DCM (25 mL) at room temperature and then cooled to 0 oC. Then, thiophosgene (11.4 g, 13.55 mmol) was added dropwise at that temperature and further stirred at room temperature for 3-4 h. After the reaction completion, the reaction mixture was diluted with water and extracted with DCM. The organic portion was dried over Na2SO4, concentrated and purified by flash column using 5% EtOAc in hexane to afford 1, 3-dichloro-2-isothiocyanatobenzene (1.75 g) in 70% yield. 1H NMR (DMSO-d6): δ 7.39 (t, J = 7.8 Hz, 1H), 7.62 (d, J = 7.8 Hz, 2H).
References:
Muthukaman, Nagarajan;Tambe, Macchindra;Deshmukh, Sanjay;Pisal, Dnyandeo;Tondlekar, Shital;Shaikh, Mahamadhanif;Sarode, Neelam;Kattige, Vidya G.;Pisat, Monali;Sawant, Pooja;Honnegowda, Srinivasa;Karande, Vikas;Kulkarni, Abhay;Behera, Dayanidhi;Jadhav, Satyawan B.;Sangana, Ramchandra R.;Gudi, Girish S.;Khairatkar-Joshi, Neelima;Gharat, Laxmikant A. [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 23,p. 5131 - 5138] Location in patent:supporting information
6579-27-7
64 suppliers
$45.00/100mg

6590-95-0
76 suppliers
$12.00/1g

6579-27-7
64 suppliers
$45.00/100mg

6590-95-0
76 suppliers
$12.00/1g
![N-[(4-Chlorophenylamino)(thiocarbonyl)]benzamide](/CAS/GIF/4921-83-9.gif)
![Benzamide, N-[[(4-chlorophenyl)amino]carbonyl]-](/CAS/20210305/GIF/57160-46-0.gif)