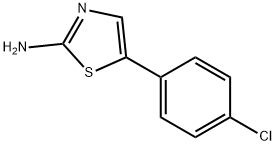
2-Amino-5-(4-chlorophenyl)thiazole synthesis
- Product Name:2-Amino-5-(4-chlorophenyl)thiazole
- CAS Number:73040-66-1
- Molecular formula:C9H7ClN2S
- Molecular Weight:210.68
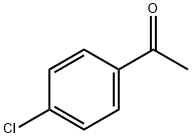
99-91-2
422 suppliers
$10.00/25g
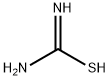
17356-08-0
3 suppliers
inquiry

73040-66-1
54 suppliers
$26.00/100mg
Yield:73040-66-1 81%
Reaction Conditions:
with iodine at 100;
Steps:
General procedure for preparation the compounds 1-3
General procedure: These compounds were prepared according to the procedurethat was described in Siddiqui et al.[17] The title compoundswere prepared by the addition of iodine (2.54 g,0.01 mol) to the 4-substituted acetophenone (0.01 mol) andthiourea (1.52 g, 0.02 mol), followed by heating of the mixtureovernight in an oil bath at 100C. After cooling, thereaction mixture was washed with diethylether (100 mL) toremove any unreacted iodine and substituted acetophenone.The solid residue was put in (200 mL) of cold water andtreated with 25% aqueous ammonium hydroxide (to pH 9-10). The precipitated thiazoles were collected and purifiedby crystallization from hot ethanol. 2-Amino-4-(4-chlorophenyl)-thiazole (1): Yield (81%);mp: 168-170C; FTIR (KBr disk cm1): 3439, 3284 symm.and asymm. NH2 group, 1633 (C D N); 1H NMR (DMSOd6,300 MHz, d): 7.81-7.79 (d, 2H, phenyl), 7.41-7.38 (d,2H, phenyl), 7.12 (s, 2H, NH2), 7.04 (s, 1H, thiazole ring);Anal. Calcd. for C9H7ClN2S (210.5 g/mol): C, 51.31; H,3.33; N, 13.30; S, 15.20. Found: C, 51.55; H, 3.39; N, 13.50;S, 15.33.
References:
Tomi, Ivan Hameed R.;Al-Daraji, Ali H. R.;Aziz, Sherien A. [Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry,2015,vol. 45,# 4,p. 605 - 613]
