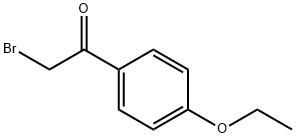
2-bromo-1-(4-ethoxyphenyl)ethanone synthesis
- Product Name:2-bromo-1-(4-ethoxyphenyl)ethanone
- CAS Number:51012-63-6
- Molecular formula:C10H11BrO2
- Molecular Weight:243.1
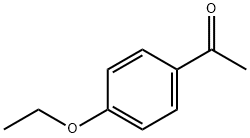
1676-63-7
261 suppliers
$6.00/1g

51012-63-6
23 suppliers
$75.00/50mg
Yield:51012-63-6 85%
Reaction Conditions:
with hydrogen bromide;potassium iodide;sodium nitrite in water at 0 - 20; for 10 h;
Steps:
General experimental procedure
General procedure: In a RBF cooled in ice bath at 0 C, HBr(12 mmol, in 2 ml of water) was taken. To this a solution of NaNO2(5 mmol, in 5ml of water) was added drop wise. The reaction was stirred for 15min maintaining the temperature at 0 °C and KI (5 mol %) was added. After 10 min ketone(10 mmol) was added at once. After 15 min reaction temperature was brought to room temperature slowly. Reaction was monitored by TLC (ethyl acetate: pet ether, 1:9). After completion of reaction 50 ml of CHCl3 was added and organic layer separated. Aqueous layer was extracted with 25 ml of CHCl3 and combined organic layer was washed with 10% NaHSO3 solution (2 x 20 ml) and 10% NaHCO3 solution (2 x 20 ml).The organic layer was dried over sodium sulphate and concentrated under reduced pressure. Pure product was obtained after column chromatography (silica gel, 60-120, eluentethyl acetate: pet ether).
References:
Ghorpade, Archana K.;Huddar, Sameerana N.;Akamanchi, Krishnacharya G. [Tetrahedron Letters,2016,vol. 57,# 44,p. 4918 - 4921] Location in patent:supporting information
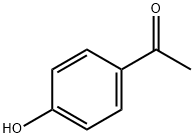
99-93-4
828 suppliers
$5.00/25g

51012-63-6
23 suppliers
$75.00/50mg
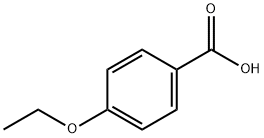
619-86-3
242 suppliers
$15.00/25g

51012-63-6
23 suppliers
$75.00/50mg