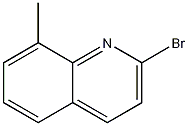
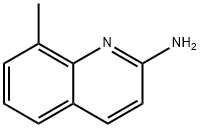
2-Bromo-8-methylquinoline synthesis
- Product Name:2-Bromo-8-methylquinoline
- CAS Number:99073-81-1
- Molecular formula:C10H8BrN
- Molecular Weight:222.08
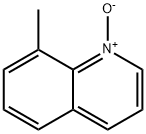
4053-38-7
28 suppliers
$87.40/1g

99073-81-1
17 suppliers
$55.00/25mg
Yield: 62%
Reaction Conditions:
with N,N-dimethyl-formamide;phosphorus(V) oxybromide in dichloromethane at 0 - 25; for 6 h;Inert atmosphere;regioselective reaction;
Steps:
9 4.2. General procedure I for the bromination of azine N-oxides
General procedure: To a stirred solution of the appropriate azine N-oxides in anhydrous CH2Cl2 (0.1 M) at 0 °C is added POBr3 (1.2 equiv) followed by dropwise addition of DMF (0.5 equiv) under argon. The resulting reaction mixture was warmed to 25 °C and stirred for several hours until the reaction is complete as indicated by TLC. Saturated aqueous sodium carbonate solution is added to the reaction mixture slowly to adjust the pH to 7-8. The resulting mixture is separated and the aqueous phase is extracted with CH2Cl2 thoroughly. The organic phase is combined and washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure to afford the crude product, which is purified by flash column chromatography using PE/EA (100:1) as eluent.
References:
Wang, Dong;Wang, Yuxi;Zhao, Junjie;Li, Linna;Miao, Longfei;Wang, Dong;Sun, Hua;Yu, Peng [Tetrahedron,2016,vol. 72,# 38,p. 5762 - 5768]
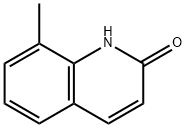
4053-36-5
62 suppliers
$35.00/1g

99073-81-1
17 suppliers
$55.00/25mg
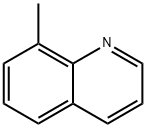
611-32-5
207 suppliers
$26.00/5g

99073-81-1
17 suppliers
$55.00/25mg
20151-45-5
14 suppliers
$45.00/10mg

99073-81-1
17 suppliers
$55.00/25mg