
2-CHLORO-1-(1-METHYL-1H-INDOL-3-YL)-ETHANONE synthesis
- Product Name:2-CHLORO-1-(1-METHYL-1H-INDOL-3-YL)-ETHANONE
- CAS Number:17716-91-5
- Molecular formula:C11H10ClNO
- Molecular Weight:207.66
603-76-9
412 suppliers
$18.00/5g

107-14-2
273 suppliers
$15.00/25g

17716-91-5
16 suppliers
$205.00/250mg
Yield:17716-91-5 53%
Reaction Conditions:
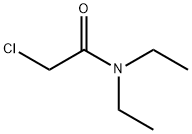
Stage #1: chloroacetonitrilewith phenylborondichloride in dichloromethane at 20; for 0.25 h;Inert atmosphere;
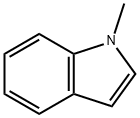
Stage #2: 1-methylindole in dichloromethane; for 1 h;Inert atmosphere;
Stage #3: with water;sodium carbonateInert atmosphere;
Steps:
General Procedure for Synthesizing 3-Acylindole Compounds (Exemplified by 3aa)
General procedure: A 1.5 mL (1.5 mmol) ofPhBCl2 (1.0 mol/L in CH2Cl2) was added to CH2Cl2 solution(4 mL) of the nitrile (5a) (173 mg, 1.2 mmol) at room temperatureunder argon. The mixture was stirred for 15 min. Theindole (1a) (131 mg, 1.0 mmol) was added dropwise to this solutionat room temperature. The resulting solution was stirredfor 3 h, and then 0.5 mol/L Na2CO3 was added to quench thereaction. The resulting mixture was extracted with CH2Cl2.The organic layer was washed with saturated brine, dried(MgSO4), and evaporated. The crude product was purifiedusing silica gel chromatography to provide 3aa (226 mg, 82%yield).
References:
Mizoi, Kenta;Mashima, Yu;Kawashima, Yuya;Takahashi, Masato;Mimori, Seisuke;Hosokawa, Masakiyo;Murakami, Yasuoki;Hamana, Hiroshi [Chemical and Pharmaceutical Bulletin,2015,vol. 63,# 7,p. 538 - 545]

603-76-9
412 suppliers
$18.00/5g

79-37-8
460 suppliers
$17.67/10gm:

17716-91-5
16 suppliers
$205.00/250mg

157427-47-9
0 suppliers
inquiry

107-14-2
273 suppliers
$15.00/25g

17716-91-5
16 suppliers
$205.00/250mg

603-76-9
412 suppliers
$18.00/5g
2315-36-8
257 suppliers
$17.00/5g

17716-91-5
16 suppliers
$205.00/250mg