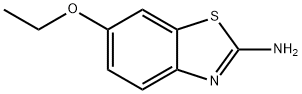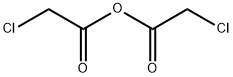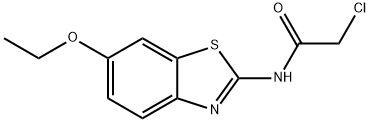
2-CHLORO-N-(6-ETHOXY-BENZOTHIAZOL-2-YL)-ACETAMIDE synthesis
- Product Name:2-CHLORO-N-(6-ETHOXY-BENZOTHIAZOL-2-YL)-ACETAMIDE
- CAS Number:3268-74-4
- Molecular formula:C11H11ClN2O2S
- Molecular Weight:270.74
Yield:3268-74-4 84%
Reaction Conditions:
with potassium carbonate in benzene;Reflux;
Steps:
5.1.3. General synthetic procedure for N-(benzo[d]thiazol-2-yl)-2-chloroacetamides (4a-k)
General procedure: Chloroacetyl chloride (0.06 mol) was added dropwise to a mixture of the appropriate 2-amino-6-Substituted benzothiazole, 3a-k (0.05 mol) and K2CO3 (0.06 mol) in benzene (50 mL) at room temperature. The reaction mixture was refluxed for 6-12 h, then, after cooling to room temperature, it was slowly poured into 100 mL of ice water. A solid was formed thereafter. The precipitate was separated by filtration and washed successively with water. The product was dried under vacuum to obtain 4a-k. The progress of the reaction was monitored by Thin Layer Chromatography using toluene: acetone (8:2) solvent system.
References:
Patel, Rahul V.;Patel, Paresh K.;Kumari, Premlata;Rajani, Dhanji P.;Chikhalia, Kishor H. [European Journal of Medicinal Chemistry,2012,vol. 53,p. 41 - 51] Location in patent:experimental part
156-43-4
238 suppliers
$5.00/5G

3268-74-4
18 suppliers
$185.63/1g

90180-69-1
0 suppliers
inquiry

3268-74-4
18 suppliers
$185.63/1g
880-29-5
63 suppliers
$12.00/1g

3268-74-4
18 suppliers
$185.63/1g