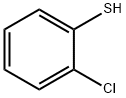
2-CHLOROTHIOPHENOL synthesis
- Product Name:2-CHLOROTHIOPHENOL
- CAS Number:6320-03-2
- Molecular formula:C6H5ClS
- Molecular Weight:144.62
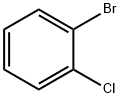
694-80-4
386 suppliers
$5.00/10g

6320-03-2
145 suppliers
$12.00/1g
Yield:6320-03-2 98.5%
Reaction Conditions:
with hydrogen sulfide at 360;Temperature;Reagent/catalyst;
Steps:
1
A stainless steel tube having an inner diameter of 25 mm and a length of 700 mm was filled with 30 ml of the catalyst obtained in Synthesis Example 1 and glass wool was filled in the upper part and lower part thereof to hold the catalyst layer to prepare a reaction tube. A back pressure valve was attached to the lower end of the reaction tube so that the internal pressure of the reaction tube could be adjusted. The reaction tube was heated to 360 ° C. in an electric furnace, and this temperature was taken as the reaction temperature of the gas phase reaction. Meanwhile, a mixture was prepared by mixing o-bromochlorobenzene (compound represented by formula (1)) at a flow rate of 0.134 mol / hr and hydrogen sulfide at a flow rate of 0.295 mol / hr to o-bromochlorobenzene The mixture was preheated and then introduced into the reaction tube to carry out a gas phase reaction. Unreacted hydrogen sulfide and by-produced hydrogen bromide gas are separated by cooling the gas after the reactionAnd the reaction solution was obtained. The composition of the reaction solution was analyzed by gas chromatography and the reaction rate of o-bromochlorobenzene and the yield of each of o-chlorothiophenol (target product), p-chlorothiophenol and m-chlorothiophenol formed was calculated.
References:
SUMITOMO SEIKA CHEMICALS COMPANY LIMITED;SUZUKI, MICHIO;SANADA, SHOHEI;YAMAMOTO, KATSUMASA JP2017/95415, 2017, A Location in patent:Paragraph 0071; 0072
95-50-1
566 suppliers
$10.00/5g

6320-03-2
145 suppliers
$12.00/1g

694-80-4
386 suppliers
$5.00/10g

6320-03-2
145 suppliers
$12.00/1g
2037-31-2
142 suppliers
$19.00/1g
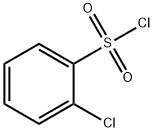
2905-23-9
198 suppliers
$6.00/1g

6320-03-2
145 suppliers
$12.00/1g
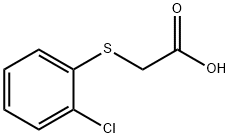
18619-18-6
16 suppliers
inquiry

6320-03-2
145 suppliers
$12.00/1g