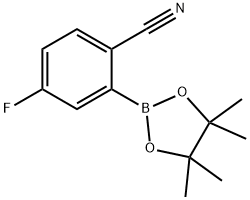
2-Cyano-5-fluorophenylboronic acid pinacol ester synthesis
- Product Name:2-Cyano-5-fluorophenylboronic acid pinacol ester
- CAS Number:463335-96-8
- Molecular formula:C13H15BFNO2
- Molecular Weight:247.07
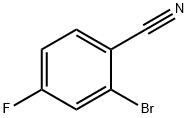
36282-26-5
196 suppliers
$7.00/5g

73183-34-3
554 suppliers
$6.00/5g

463335-96-8
39 suppliers
$55.00/250mg
Yield:463335-96-8 88%
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane;dimethyl sulfoxide at 85; for 20 h;
Steps:
4 EXAMPLE 4 4-Fluoro-2-{6-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo[1,2-a]pyridin- [3-YLLPYRIDIN-2-YLTBENZONITRILE]
A mixture of 4-fluoro-2-bromobenzonitrile (10.0 g, 50.0 mmol), potassium acetate (9.82 g, 100 mmol), bis [(PINACOLATO)] diboron (14.0 g, 55.0 mmol) and dichloro [[L,] [1'-BIS] (diphenylphosphino) ferrocene] palladium (II) dichloromethane adduct (0.82 g, 1.0 mmol) in 1, 4-dioxane (150 ml containing 3 ml dimethylsulfoxide) was degassed with nitrogen for 1 h then heated at [85°C] for 20 h. The reaction was cooled to ambient temperature and then concentrated [I71] uacuo. The residue was stirred with 2N sodium hydroxide (250 ml) for 10 min then filtered. The filtrate was extracted with diethyl ether (300 ml) and the organics discarded. The aqueous component was cooled to [0°C] then treated with 5N hydrochloric acid added dropwise over 15 min until pH 8. The aqueous phase was extracted with ethyl acetate (2 x 200 ml), the combined organics were dried over anhydrous sodium sulfate, filtered and evaporated to afford 4- [FLUORO-2- (4,] 4,5, 5-tetramethyl- [1,3, 2] dioxaborolan-2-yl) benzonitrile (10.9 g, [88%)] as a pale brown solid: [ON] (360 MHz, CDCl3) 1. 38 (12H, s), 7.15-7. 25 [(1H,] m), 7.53-7. 60 [(1H,] m), 7.67-7. 75 [(1H,] m). 2,6-Dibromopyridine (0.94 g, 4.0 mmol), [4-FLUORO-2- (4,] 4,5, [5-] tetramethyl- [1, 3,2] dioxaborolan-2-yl) benzonitrile (1.48 g, 6.0 mmol) and potassium phosphate (1.70 g, 8. 0 mmol) were dissolved in [N, N-] dimethylformamide (12 ml) and degassed with nitrogen for 15 min. Tetrakis (triphenylphosphine) palladium (0) (230 mg, 0.2 mmol) was added then the mixture heated at [80°C] for 16 h. The mixture was allowed to cool to ambient temperature, diluted with water (150 ml) and extracted into ethyl acetate (2 x 150 ml). The combined organics were washed with brine (100 ml), dried over anhydrous sodium sulfate and evaporated to give a yellow oil. Purification by flash column chromatography on silica eluting with isohexane on a gradient of ethyl acetate (10-15%) gave 4-fluoro-2- (6- [BROMOPYRIDIN-2-YL) BENZONITRILE] (0.42 g, 38%) as a waxy solid: [SN] (360 MHz, CDCl3) 7.21-7. 25 [(1H,] m), 7.59 [(1H,] d, J 8), 7.67 [(1H,] dd, [J 9.] 3,2. 6), 7.72 [(1H,] t, J 7.7), 7.78-7. 87 (2H, m). A mixture [OF 2-(8-FLUOROIMIDAZO [1, 2-A] PYRIDIN-7-YL)] propan-2-ol (97 mg, 0.5 mmol), [4-FLUORO-2-(6-BROMOPYRIDIN-2-YL)] benzonitrile (166 mg, 0.6 mmol) and [CS2CO3] [(538] mg, 1.65 mmol) in 1,4-dioxane (4 ml) was degassed with nitrogen for 30 min. Tetrakis (triphenylphosphine)- palladium [(0)] (29 mg, 0.03 mmol) was added and the mixture heated under reflux for 56 h. On cooling, the mixture was partitioned between ethyl acetate (100 ml) and water (100 ml). The organics were washed with water (100 [ML)] and brine (50 ml), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo to give an orange oil. The oil was purified by flash column chromatography on silica, eluting with dichloromethane (+1% 0. 880 ammonia solution) on a gradient of methanol (2-3%). Collecting appropriate fractions followed by trituration with diethyl ether (5 ml) gave the title imidazopyridine as a white amorphous solid (131 mg, 67%): [SN] (400 MHz, [CD13)] 1.74 (6H, d, J 1.2), 2.07 [(1H,] s), 7.26-7. 30 (2H, m), 7.56-7. 63 (2H, m), 7. [85-7.] 94 (3H, m), 8.22 [(1H,] [S),] 9.74 [(1H,] d, [J 7.] 4); [IN/Z] (ES+) 391 (100%, [MH] +).
References:
WO2003/99817,2003,A1 Location in patent:Page 39-40
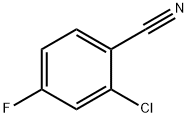
60702-69-4
283 suppliers
$10.00/5g

73183-34-3
554 suppliers
$6.00/5g

463335-96-8
39 suppliers
$55.00/250mg

36282-26-5
196 suppliers
$7.00/5g

463335-96-8
39 suppliers
$55.00/250mg