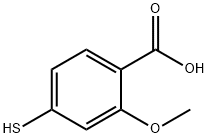
2-METHOXY-4-MERCAPTOBENZOIC ACID synthesis
- Product Name:2-METHOXY-4-MERCAPTOBENZOIC ACID
- CAS Number:95420-72-7
- Molecular formula:C8H8O3S
- Molecular Weight:184.21
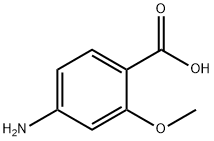
2486-80-8
125 suppliers
$11.00/500mg
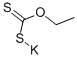
140-89-6
302 suppliers
$12.00/5g

95420-72-7
14 suppliers
$85.00/5mg
Yield:95420-72-7 78%
Reaction Conditions:
Stage #1: 4-amino-2-methoxybenzoic acidwith hydrogenchloride;sodium nitrite in water at 0;
Stage #2: potassium ethyl xanthogenate in water at 0 - 20; for 1 h;
Stage #3: with sodium hydroxide in ethanol; for 7 h;Reflux;
Steps:
2-Methoxy-4-sulfanylbenzoic acid (15)
b. A solution of 3.0 g (17.96 mmol) of amine 18 in7 mL of conc. HCl, cooled to 0°, was treated with a solution of 1.31 g (19.0 mmol) of NaNO2 in 5 mL of water. Potassium ethyl xanthate, 2.8 g (20 mmol), was then added to the resulting diazonium salt solution.The mixture was stirred at 20° for 1 h, and the crude xanthate that precipitated was filtered off. The precipitate was dissolved in 25 mL of ethanol, and 1.4 g(35.0 mmol) of NaOH was added. The mixture was heated under reflux for 7 h, after which the alcohol was removed by distillation, and the dry residue was acidified with 10% AcOH and filtered off. The product was extracted with heptane. Yield 78%, mp 96-97°.1 NMR spectrum (CDCl3), δ, ppm: 3.73 s (1, SH),4.06 s (3, 3), 6.90 s (1, 3), 6.98 d (1, 5, J8.0 Hz), 8.00 d (1, 6, J 8.0 Hz), 10.64 br.s (1,COOH). 13 NMR spectrum (CDCl3), δ, ppm: 56.9(3), 111.0 (5), 114.5 (1), 121.9 (3), 134.1 (6),141.3 (4), 158.2 (2), 165.6 (COOH). Found, %: 52.05; 4.40. C8H8O3S. Calculated, %: C 52.16; 4.38.
References:
Lomov [Russian Journal of Organic Chemistry,2019,vol. 55,# 8,p. 1093 - 1098][Zh. Org. Khim.,2019,vol. 55,# 8,p. 1182 - 1187,6]

89469-32-9
10 suppliers
inquiry

95420-72-7
14 suppliers
$85.00/5mg
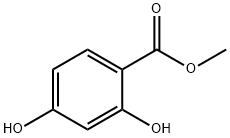
2150-47-2
229 suppliers
$7.00/5g

95420-72-7
14 suppliers
$85.00/5mg

28478-45-7
13 suppliers
inquiry

95420-72-7
14 suppliers
$85.00/5mg

5427-29-2
19 suppliers
inquiry

95420-72-7
14 suppliers
$85.00/5mg